Treating impulsivity with probiotics in adults (PROBIA): study protocol of a multicenter, double-blind, randomized, placebo-controlled trial
- PMID: 32046750
- PMCID: PMC7014653
- DOI: 10.1186/s13063-019-4040-x
Treating impulsivity with probiotics in adults (PROBIA): study protocol of a multicenter, double-blind, randomized, placebo-controlled trial
Abstract
Background: Impulsivity and compulsivity are related to emotional and social maladjustment and often underlie psychiatric disorders. Recently, alterations in microbiota composition have been shown to have implications for brain development and social behavior via the microbiota-gut-brain axis. However, the exact mechanisms are not fully identified. Recent evidence suggests the modulatory effect of synbiotics on gut microbiota and the contribution of these agents in ameliorating symptoms of many psychiatric diseases. To date, no randomized controlled trial has been performed to establish the feasibility and efficacy of this intervention targeting the reduction of impulsivity and compulsivity. We hypothesize that supplementation with synbiotics may be an effective treatment in adults with high levels of impulsivity and/or compulsivity.
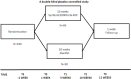
Methods/design: This is a prospective, multicenter, double-blind, randomized controlled trial with two arms: treatment with a synbiotic formula versus placebo treatment. The primary outcome is the response rate at the end of the placebo-controlled phase (response defined as a Clinical Global Impression-Improvement Scale score of 1 or 2 = very much improved or much improved, plus a reduction in the Affective Reactivity Index total score of at least 30% compared with baseline). A total of 180 participants with highly impulsive behavior and a diagnosis of attention deficit/hyperactivity disorder (ADHD) and/or borderline personality disorder, aged 18-65 years old, will be screened at three study centers. Secondary outcome measures, including changes in general psychopathology, ADHD symptoms, neurocognitive function, somatic parameters, physical activity, nutritional intake, and health-related quality of life, will be explored at assessments before, during, and at the end of the intervention. The effect of the intervention on genetics, microbiota, and several blood biomarkers will also be assessed. Gastrointestinal symptoms and somatic complaints will additionally be explored at 1-week follow-up.
Discussion: This is the first randomized controlled trial to determine the effects of supplementation with synbiotics on reducing impulsive and compulsive behavior. This clinical trial can contribute to explaining the mechanisms involved in the crosstalk between the intestinal microbiome and the brain. If effects can be established by reducing impulsive and compulsive behavior, new cost-effective treatments might become available to these patients.
Trial registration: ClinicalTrials.gov, NCT03495375. Registered on 26 February 2018.
Keywords: ADHD; Aggression; Borderline personality disorder; Compulsivity; Impulsivity; Microbiome; Nutrition; Probiotics; Synbiotics.
Conflict of interest statement
IB has served as an advisor/consultant in the last 5 years for Angelini, Eli Lilly, Gedeon Richter, and Pierre Fabre and has lectured for Eli Lilly, Janssen, Lundbeck, Gedeon Richter, and Servier. JARQ was on the speaker's bureau and/or acted as consultant for Eli Lilly, Janssen-Cilag, Novartis, Shire, Takeda, Bial, Shionogui, Lundbeck, Almirall, Braingaze, Sincrolab, Medice and Rubio in the last 5 years. He has also received travel awards (air-tickets + hotel accomodation) for taking part in psychiatric meetings organized by Janssen-Cilag, Rubio, Shire, Medice and Eli Lilly. The Department of Psychiatry, chaired by him, has received unrestricted educational and research support from Eli Lilly, Lundbeck, Janssen-Cilag, Actelion, Shire, Ferrer, Oryzon, Roche, Psious and Rubio in the last 5 years. YG has received speaker fees, has received reimbursement for travel costs, and/or has served as a consultat for Novartis, HB Pharma, Shire, Eli Lilly, Hogrefe, Broadman, Clarke Partners, Medscape, Medibas, and Natur & Kultur. All other authors declare that they have no competing interests.
References
-
- Potenza MN. To do or not to do? The complexities of addiction, motivation, self-control, and impulsivity. Am J Psychiatry. 2007;164(1):4–6. - PubMed
-
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20(3):255–261. - PubMed
-
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163(7):1282–1284. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical