A randomized controlled trial protocol comparing the feeds of fresh versus frozen mother's own milk for preterm infants in the NICU
- PMID: 32046760
- PMCID: PMC7014600
- DOI: 10.1186/s13063-019-3981-4
A randomized controlled trial protocol comparing the feeds of fresh versus frozen mother's own milk for preterm infants in the NICU
Abstract
Background: Necrotizing enterocolitis (NEC) is the leading cause of death among preterm infants born at < 30 weeks' gestation. The incidence of NEC is reduced when infants are fed human milk. However, in many neonatal intensive care units (NICUs), it is standard practice to freeze and/or pasteurize human milk, which deactivates bioactive components that may offer additional protective benefits. Indeed, our pilot study showed that one feed of fresh mother's own milk per day was safe, feasible, and can reduce morbidity in preterm infants. To further evaluate the benefits of fresh human milk in the NICU, a randomized controlled trial is needed.
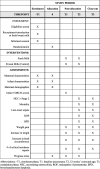
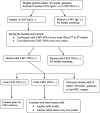
Methods: Our prospective multicenter, double-blinded, randomized, controlled trial will include infants born at < 30 weeks' gestation and admitted to one of 29 tertiary NICUs in China. Infants in the intervention (fresh human milk) group (n = 1549) will receive at least two feeds of fresh human milk (i.e., within 4 h of expression) per day from the time of enrollment until 32 weeks' corrected age or discharge to home. Infants in the control group (n = 1549) will receive previously frozen human milk following the current standard protocols. Following informed consent, enrolled infants will be randomly allocated to the control or fresh human milk groups. The primary outcome is the composite outcome mortality or NEC ≥ stage 2 at 32 weeks' corrected age, and the secondary outcomes are mortality, NEC ≥ stage 2, NEC needing surgery, late-onset sepsis, retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), weight gain, change in weight, increase in length, increase in head circumference, time to full enteral feeds, and finally, the number and type of critical incident reports, including feeding errors.
Discussion: Our double-blinded, randomized, controlled trial aims to examine whether fresh human milk can improve infant outcomes. The results of this study will impact both Chinese and international medical practice and feeding policy for preterm infants. In addition, data from our study will inform changes in health policy in NICUs across China, such that mothers are encouraged to enter the NICU and express fresh milk for their infants.
Trial registration: Chinese Clinical Trial Registry; #ChiCTR1900020577; registered January 1, 2019; http://www.chictr.org.cn/showprojen.aspx?proj=34276.
Keywords: Breast milk, Preterm, Necrotizing enterocolitis, Neonatal intensive care unit (NICU).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Testing the feasibility and safety of feeding preterm infants fresh mother's own milk in the NICU: A pilot study.Sci Rep. 2019 Jan 30;9(1):941. doi: 10.1038/s41598-018-37111-7. Sci Rep. 2019. PMID: 30700726 Free PMC article. Clinical Trial.
-
Two speeds of increasing milk feeds for very preterm or very low-birthweight infants: the SIFT RCT.Health Technol Assess. 2020 Apr;24(18):1-94. doi: 10.3310/hta24180. Health Technol Assess. 2020. PMID: 32342857 Free PMC article. Clinical Trial.
-
Oropharyngeal administration of mother's colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial.Trials. 2015 Oct 12;16:453. doi: 10.1186/s13063-015-0969-6. Trials. 2015. PMID: 26458907 Free PMC article. Clinical Trial.
-
Probiotics for prevention of necrotizing enterocolitis in preterm infants.Evid Based Child Health. 2014 Sep;9(3):584-671. doi: 10.1002/ebch.1976. Evid Based Child Health. 2014. PMID: 25236307 Review.
-
Early enteral feeding in preterm infants.Semin Perinatol. 2019 Nov;43(7):151159. doi: 10.1053/j.semperi.2019.06.007. Epub 2019 Jul 24. Semin Perinatol. 2019. PMID: 31443906 Review.
Cited by
-
Early versus delayed enteral nutrition for neonatal hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia: a randomized controlled trial.Ital J Pediatr. 2022 Aug 15;48(1):146. doi: 10.1186/s13052-022-01342-2. Ital J Pediatr. 2022. PMID: 35971138 Free PMC article. Clinical Trial.
-
Impact of Storage Conditions on the Breast Milk Peptidome.Nutrients. 2020 Sep 8;12(9):2733. doi: 10.3390/nu12092733. Nutrients. 2020. PMID: 32911625 Free PMC article.
-
Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development.Biomolecules. 2021 Jun 7;11(6):851. doi: 10.3390/biom11060851. Biomolecules. 2021. PMID: 34200323 Free PMC article. Review.
-
Research progress on pathophysiologic mechanisms, clinical treatment and predictive biomarkers in bronchopulmonary dysplasia: from the perspective of oxidative stress.Front Pediatr. 2025 Mar 27;12:1343870. doi: 10.3389/fped.2024.1343870. eCollection 2024. Front Pediatr. 2025. PMID: 40212666 Free PMC article. Review.
-
Dose-dependent effect of human milk on Bronchopulmonary dysplasia in very low birth weight infants.BMC Pediatr. 2020 Nov 16;20(1):522. doi: 10.1186/s12887-020-02394-1. BMC Pediatr. 2020. PMID: 33190629 Free PMC article.
References
-
- e-Library of Evidence for Nutrition Actions (eLENA): mother’s milk for low-birth-weight infants. 2019. https://www.who.int/elena/titles/mothersmilk_infants/en/. Accessed 7 May 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

