Dissection of the cecal microbial community in chickens after Eimeria tenella infection
- PMID: 32046772
- PMCID: PMC7014781
- DOI: 10.1186/s13071-020-3897-6
Dissection of the cecal microbial community in chickens after Eimeria tenella infection
Abstract
Background: Eimeria spp. are responsible for chicken coccidiosis which is the most important enteric protozoan disease resulting in tremendous economic losses in the poultry industry. Understanding the interaction between the avian cecal microbiota and coccidia is of interest in the development of alternative treatments that do not rely on chemotherapeutics and do not lead to drug resistance.
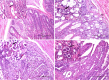
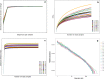
Methods: We utilized 16S rRNA gene sequencing to detect the dynamics of the cecal microbial community in AA broilers challenged with Eimeria tenella. Histopathological analysis of the cecum was also conducted.
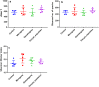
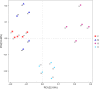
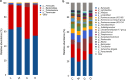
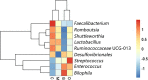
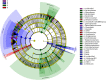
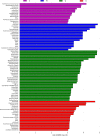
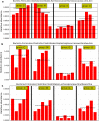
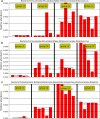
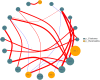
Results: We found that microbial shifts occur during the infection. Lactobacillus, Faecalibacterium, Ruminococcaceae UCG-013, Romboutsia and Shuttleworthia decreased in abundance. However, the opportunistic pathogens Enterococcus and Streptococcus increased in abundance over time in response to the infection.
Conclusions: Eimeria tenella disrupts the integrity of the cecal microbiota and could promote the establishment and growth of potentially pathogenic bacteria. Defining bacterial populations affected by coccidial infection might help identify bacterial markers for intestinal disease as well as populations or species that could be beneficial in maintaining and restoring gut homeostasis during and after infection with E. tenella.
Keywords: 16S rRNA; Alternative therapeutics; Cecal microbiota; Chicken coccidiosis; Eimeria tenella.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Eimeria tenella infection perturbs the chicken gut microbiota from the onset of oocyst shedding.Vet Parasitol. 2018 Jul 15;258:30-37. doi: 10.1016/j.vetpar.2018.06.005. Epub 2018 Jun 5. Vet Parasitol. 2018. PMID: 30105975
-
Susceptibility and cecal microbiota alteration to Eimeria-infection in Yellow-feathered broilers, Arbor Acres broilers and Lohmann pink layers.Poult Sci. 2024 Jul;103(7):103824. doi: 10.1016/j.psj.2024.103824. Epub 2024 May 7. Poult Sci. 2024. PMID: 38772089 Free PMC article.
-
Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum.Poult Sci. 2020 Mar;99(3):1297-1305. doi: 10.1016/j.psj.2019.10.073. Epub 2020 Jan 22. Poult Sci. 2020. PMID: 32111306 Free PMC article.
-
Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: a review.Parasite. 2021;28:48. doi: 10.1051/parasite/2021047. Epub 2021 Jun 2. Parasite. 2021. PMID: 34076575 Free PMC article. Review.
-
Botanicals: A promising approach for controlling cecal coccidiosis in poultry.Front Vet Sci. 2023 Apr 25;10:1157633. doi: 10.3389/fvets.2023.1157633. eCollection 2023. Front Vet Sci. 2023. PMID: 37180056 Free PMC article. Review.
Cited by
-
Assessing the impact of hatching system and body weight on the growth performance, caecal short-chain fatty acids, and microbiota composition and functionality in broilers.Anim Microbiome. 2024 Jul 24;6(1):41. doi: 10.1186/s42523-024-00331-6. Anim Microbiome. 2024. PMID: 39049129 Free PMC article.
-
Histopathologic observations in a coccidiosis model of Eimeria tenella.Animal Model Exp Med. 2024 Dec;7(6):893-903. doi: 10.1002/ame2.12463. Epub 2024 Oct 22. Animal Model Exp Med. 2024. PMID: 39439067 Free PMC article.
-
Effect of Dietary Benzoic Acid and Oregano Essential Oil as a Substitute for an Anti-Coccidial Agent on Growth Performance and Physiological and Immunological Responses in Broiler Chickens Challenged with Eimeria Species.Animals (Basel). 2024 Oct 17;14(20):3008. doi: 10.3390/ani14203008. Animals (Basel). 2024. PMID: 39457937 Free PMC article.
-
Natural Magnolol ameliorates coccidiosis infected with Eimeria tenella by affecting antioxidant, anti-inflammatory, and gut microbiota of chicks.Poult Sci. 2023 Nov;102(11):102975. doi: 10.1016/j.psj.2023.102975. Epub 2023 Aug 2. Poult Sci. 2023. PMID: 37708766 Free PMC article.
-
Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection.Microorganisms. 2022 Jul 31;10(8):1548. doi: 10.3390/microorganisms10081548. Microorganisms. 2022. PMID: 36013966 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

