Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020
- PMID: 32046815
- PMCID: PMC7029448
- DOI: 10.2807/1560-7917.ES.2020.25.6.2000082
Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020
Abstract
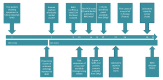
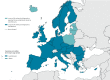
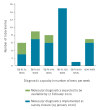
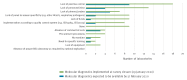
Timely detection of novel coronavirus (2019-nCoV) infection cases is crucial to interrupt the spread of this virus. We assessed the required expertise and capacity for molecular detection of 2019-nCoV in specialised laboratories in 30 European Union/European Economic Area (EU/EEA) countries. Thirty-eight laboratories in 24 EU/EEA countries had diagnostic tests available by 29 January 2020. A coverage of all EU/EEA countries was expected by mid-February. Availability of primers/probes, positive controls and personnel were main implementation barriers.
Keywords: 2019-nCoV; coronavirus; emerging infections; laboratory; response; zoonoses.
Conflict of interest statement
Figures
References
-
- Novel 2019 coronavirus genome. [Accessed 02 Feb 2020]. Available from: http://virological.org/t/novel-2019-coronavirus-genome/319
-
- European Centre for Disease Control and Prevention (ECDC). Novel coronavirus. Stockholm: ECDC. [Accessed 11 Feb 2020]. Available from: https://www.ecdc.europa.eu/en/novel-coronavirus-china
-
- World Health Organization (WHO). Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Geneva: WHO; 30 Jan 2020. [Accessed 01 Feb 2020], Available from: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
