Placebo response in trials of drug treatments for cancer-related fatigue: a systematic review, meta-analysis and meta-regression
- PMID: 32046962
- PMCID: PMC7691807
- DOI: 10.1136/bmjspcare-2019-002163
Placebo response in trials of drug treatments for cancer-related fatigue: a systematic review, meta-analysis and meta-regression
Abstract
Background: Cancer-related fatigue (CRF) is one of the most distressing symptoms experienced by patients. There is no gold standard treatment, although multiple drugs have been tested with little evidence of efficacy. Randomised controlled trials (RCTs) of these drugs have commented on the existence or size of the placebo response (PR). The objective of this systematic review was to establish the magnitude of the PR in RCTs of drugs to relieve CRF and to identify contributing factors.
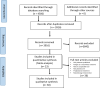
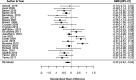
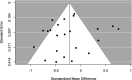
Method: RCTs were included in which the objective was to treat CRF. A meta-analysis was conducted using the standardised mean change (SMC) between baseline and final measurement in the placebo group. To explore factors that may be associated with the PR (eg, population or drug), a meta-regression was undertaken. Risk of bias was assessed using the revised Cochrane tool.
Results: From 3916 citations, 30 relevant RCTs were identified. All had limitations that increased their risk of bias. The pooled SMC in reduction in fatigue status in placebo groups was -0.23 (95% confidence intervals -0.42 to -0.04). None of the variables analysed in the meta-regression were statistically significant related to PR.
Conclusion: There is some evidence, based on trials with small samples, that the PR in trials testing drugs for CRF is non-trivial in size and statistically significant. We recommend that researchers planning drug studies in CRF should consider implementing alternative trial designs to better account for PR and decrease impact on the study results.
Keywords: cancer; fatigue; methodological research.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials