Pericyte-Mediated Tissue Repair through PDGFRβ Promotes Peri-Infarct Astrogliosis, Oligodendrogenesis, and Functional Recovery after Acute Ischemic Stroke
- PMID: 32046974
- PMCID: PMC7070447
- DOI: 10.1523/ENEURO.0474-19.2020
Pericyte-Mediated Tissue Repair through PDGFRβ Promotes Peri-Infarct Astrogliosis, Oligodendrogenesis, and Functional Recovery after Acute Ischemic Stroke
Abstract
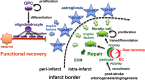
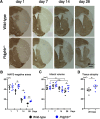
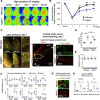
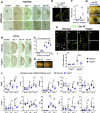
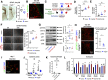
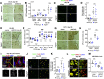
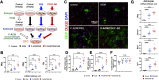
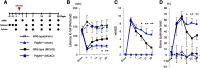
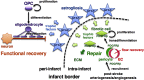
Post-stroke functional recovery can occur spontaneously during the subacute phase; however, how post-stroke fibrotic repair affects functional recovery is highly debated. Platelet-derived growth factor receptor β (PDGFRβ)-expressing pericytes are responsible for post-stroke fibrotic repair within infarct areas; therefore, we examined peri-infarct neural reorganization and functional recovery after permanent middle cerebral artery occlusion (pMCAO) using pericyte-deficient Pdgfrb+/- mice. Time-dependent reduction of infarct area sizes, i.e., repair, was significantly impaired in Pdgfrb+/- mice with recovery of cerebral blood flow (CBF) in ischemic areas attenuated by defective leptomeningeal arteriogenesis and intrainfarct angiogenesis. Peri-infarct astrogliosis, accompanied by increased STAT3 phosphorylation, was attenuated in Pdgfrb+/- mice. Pericyte-conditioned medium (PCM), particularly when treated with platelet-derived growth factor subunit B (PDGFB) homodimer (PDGF-BB; PCM/PDGF-BB), activated STAT3 and enhanced the proliferation and activity of cultured astrocytes. Although peri-infarct proliferation of oligodendrocyte (OL) precursor cells (OPCs) was induced promptly after pMCAO regardless of intrainfarct repair, OPC differentiation and remyelination were significantly attenuated in Pdgfrb+/- mice. Consistently, astrocyte-CM (ACM) promoted OPC differentiation and myelination, which were enhanced remarkably by adding PCM/PDGF-BB to the medium. Post-stroke functional recovery correlated well with the extent and process of intrainfarct repair and peri-infarct oligodendrogenesis. Overall, pericyte-mediated intrainfarct fibrotic repair through PDGFRβ may promote functional recovery through enhancement of peri-infarct oligodendrogenesis as well as astrogliosis after acute ischemic stroke.
Keywords: astrocyte; neurorestoration; oligodendrogenesis; pericyte; platelet-derived growth factor receptor β; repair.
Copyright © 2020 Shibahara et al.
Figures

Similar articles
-
Reciprocal Interaction Between Pericytes and Macrophage in Poststroke Tissue Repair and Functional Recovery.Stroke. 2020 Oct;51(10):3095-3106. doi: 10.1161/STROKEAHA.120.029827. Epub 2020 Sep 16. Stroke. 2020. PMID: 32933419
-
PDGFRβ-positive cell-mediated post-stroke remodeling of fibronectin and laminin α2 for tissue repair and functional recovery.J Cereb Blood Flow Metab. 2023 Apr;43(4):518-530. doi: 10.1177/0271678X221145092. Epub 2022 Dec 14. J Cereb Blood Flow Metab. 2023. PMID: 36514952 Free PMC article.
-
Possible involvement of basic FGF in the upregulation of PDGFRβ in pericytes after ischemic stroke.Brain Res. 2016 Jan 1;1630:98-108. doi: 10.1016/j.brainres.2015.11.003. Epub 2015 Nov 10. Brain Res. 2016. PMID: 26569132
-
Pericyte-Mediated Molecular Mechanisms Underlying Tissue Repair and Functional Recovery after Ischemic Stroke.J Atheroscler Thromb. 2023 Sep 1;30(9):1085-1094. doi: 10.5551/jat.RV22007. Epub 2023 Jun 30. J Atheroscler Thromb. 2023. PMID: 37394570 Free PMC article. Review.
-
Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function.Neurochem Int. 2017 Jul;107:88-103. doi: 10.1016/j.neuint.2016.12.016. Epub 2017 Jan 3. Neurochem Int. 2017. PMID: 28057555 Review.
Cited by
-
Isolation methods and characterization of primary rat neurovascular cells.J Biol Eng. 2024 Jul 11;18(1):39. doi: 10.1186/s13036-024-00434-3. J Biol Eng. 2024. PMID: 38992711 Free PMC article.
-
Targeting the neurovascular unit: Therapeutic potential of traditional Chinese medicine for the treatment of stroke.Heliyon. 2024 Sep 20;10(19):e38200. doi: 10.1016/j.heliyon.2024.e38200. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39386825 Free PMC article. Review.
-
The Pre-Stroke Induction and Normalization of Insulin Resistance Respectively Worsens and Improves Functional Recovery.Int J Mol Sci. 2023 Feb 16;24(4):3989. doi: 10.3390/ijms24043989. Int J Mol Sci. 2023. PMID: 36835405 Free PMC article.
-
Protein Nanoparticles Modified with PDGF-B as a Novel Therapy After Acute Cerebral Infarction.eNeuro. 2021 Sep 15;8(5):ENEURO.0098-21.2021. doi: 10.1523/ENEURO.0098-21.2021. Print 2021 Sep-Oct. eNeuro. 2021. PMID: 34462309 Free PMC article.
-
Pericytes for Therapeutic Approaches to Ischemic Stroke.Front Neurosci. 2021 Jun 18;15:629297. doi: 10.3389/fnins.2021.629297. eCollection 2021. Front Neurosci. 2021. PMID: 34239409 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
