Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis
- PMID: 32046998
- PMCID: PMC7299787
- DOI: 10.1158/1078-0432.CCR-19-3492
Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis
Abstract
Purpose: While various studies have highlighted the prognostic significance of pathologic complete response (pCR) after neoadjuvant chemotherapy (NAT), the impact of additional adjuvant therapy after pCR is not known.
Experimental design: PubMed was searched for studies with NAT for breast cancer and individual patient-level data was extracted for analysis using plot digitizer software. HRs, with 95% probability intervals (PI), measuring the association between pCR and overall survival (OS) or event-free survival (EFS), were estimated using Bayesian piece-wise exponential proportional hazards hierarchical models including pCR as predictor.
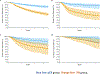
Results: Overall, 52 of 3,209 publications met inclusion criteria, totaling 27,895 patients. Patients with a pCR after NAT had significantly better EFS (HR = 0.31; 95% PI, 0.24-0.39), particularly for triple-negative (HR = 0.18; 95% PI, 0.10-0.31) and HER2+ (HR = 0.32; 95% PI, 0.21-0.47) disease. Similarly, pCR after NAT was also associated with improved survival (HR = 0.22; 95% PI, 0.15-0.30). The association of pCR with improved EFS was similar among patients who received subsequent adjuvant chemotherapy (HR = 0.36; 95% PI, 0.19-0.67) and those without adjuvant chemotherapy (HR = 0.36; 95% PI, 0.27-0.54), with no significant difference between the two groups (P = 0.60).
Conclusions: Achieving pCR following NAT is associated with significantly better EFS and OS, particularly for triple-negative and HER2+ breast cancer. The similar outcomes with or without adjuvant chemotherapy in patients who attain pCR likely reflects tumor biology and systemic clearance of micrometastatic disease, highlighting the potential of escalation/deescalation strategies in the adjuvant setting based on neoadjuvant response.See related commentary by Esserman, p. 2771.
©2020 American Association for Cancer Research.
Conflict of interest statement
No other authors have any relevant conflicts of interest.
Figures




Comment in
-
Personalization of Treatment is the Way Forward in Care and Trials.Clin Cancer Res. 2020 Jun 15;26(12):2771-2773. doi: 10.1158/1078-0432.CCR-20-0604. Epub 2020 Apr 7. Clin Cancer Res. 2020. PMID: 32265219
Comment on
-
Personalization of Treatment is the Way Forward in Care and Trials.Clin Cancer Res. 2020 Jun 15;26(12):2771-2773. doi: 10.1158/1078-0432.CCR-20-0604. Epub 2020 Apr 7. Clin Cancer Res. 2020. PMID: 32265219
References
-
- Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant Versus Adjuvant Systemic Treatment in Breast Cancer: A Meta-Analysis. J Natl Cancer Inst 2005; 97: 188–94. - PubMed
-
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008; 26: 778–85. - PubMed
-
- Bardia A, Baselga J. Neoadjuvant therapy as a platform for drug development and approval in breast cancer. Clin Cancer Res 2013; 19: 6360–70. - PubMed
-
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 2014; 384: 164–72. - PubMed
-
- Berruti A, Amoroso V, Gallo F, et al. Pathologic Complete Response As a Potential Surrogate for the Clinical Outcome in Patients With Breast Cancer After Neoadjuvant Therapy: A Meta-Regression of 29 Randomized Prospective Studies. J Clin Oncol 2014; 32: 3883–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

