Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination
- PMID: 32047033
- PMCID: PMC7049170
- DOI: 10.1073/pnas.1909814117
Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination
Abstract
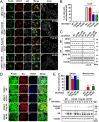
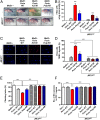
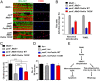
VDAC1 is a critical substrate of Parkin responsible for the regulation of mitophagy and apoptosis. Here, we demonstrate that VDAC1 can be either mono- or polyubiquitinated by Parkin in a PINK1-dependent manner. VDAC1 deficient with polyubiquitination (VDAC1 Poly-KR) hampers mitophagy, but VDAC1 deficient with monoubiquitination (VDAC1 K274R) promotes apoptosis by augmenting the mitochondrial calcium uptake through the mitochondrial calcium uniporter (MCU) channel. The transgenic flies expressing Drosophila Porin K273R, corresponding to human VDAC1 K274R, show Parkinson disease (PD)-related phenotypes including locomotive dysfunction and degenerated dopaminergic neurons, which are relieved by suppressing MCU and mitochondrial calcium uptake. To further confirm the relevance of our findings in PD, we identify a missense mutation of Parkin discovered in PD patients, T415N, which lacks the ability to induce VDAC1 monoubiquitination but still maintains polyubiquitination. Interestingly, Drosophila Parkin T433N, corresponding to human Parkin T415N, fails to rescue the PD-related phenotypes of Parkin-null flies. Taken together, our results suggest that VDAC1 monoubiquitination plays important roles in the pathologies of PD by controlling apoptosis.
Keywords: PINK1; Parkin; Parkinson disease; VDAC1; apoptosis.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Baba Y., et al. , Phenotypic commonalities in familial and sporadic Parkinson disease. Arch. Neurol. 63, 579–583 (2006). - PubMed
-
- Bonifati V., et al. , Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 (2003). - PubMed
-
- Hague S., et al. , Early-onset Parkinson’s disease caused by a compound heterozygous DJ-1 mutation. Ann. Neurol. 54, 271–274 (2003). - PubMed
-
- Hatano Y., et al. , Novel PINK1 mutations in early-onset parkinsonism. Ann. Neurol. 56, 424–427 (2004). - PubMed
-
- Kitada T., et al. , Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

