CDKG1 Is Required for Meiotic and Somatic Recombination Intermediate Processing in Arabidopsis
- PMID: 32047050
- PMCID: PMC7145484
- DOI: 10.1105/tpc.19.00942
CDKG1 Is Required for Meiotic and Somatic Recombination Intermediate Processing in Arabidopsis
Abstract
The Arabidopsis (Arabidopsis thaliana) cyclin-dependent kinase G1 (CDKG1) is necessary for recombination and synapsis during male meiosis at high ambient temperature. In the cdkg1-1 mutant, synapsis is impaired and there is a dramatic reduction in the number of class I crossovers, resulting in univalents at metaphase I and pollen sterility. Here, we demonstrate that CDKG1 is necessary for the processing of recombination intermediates in the canonical ZMM recombination pathway and that loss of CDKG1 results in increased class II crossovers. While synapsis and events associated with class I crossovers are severely compromised in a cdkg1-1 mutant, they can be restored by increasing the number of recombination intermediates in the double cdkg1-1 fancm-1 mutant. Despite this, recombination intermediates are not correctly resolved, leading to the formation of chromosome aggregates at metaphase I. Our results show that CDKG1 acts early in the recombination process and is necessary to stabilize recombination intermediates. Finally, we show that the effect on recombination is not restricted to meiosis and that CDKG1 is also required for normal levels of DNA damage-induced homologous recombination in somatic tissues.
© 2020 American Society of Plant Biologists. All rights reserved.
Figures
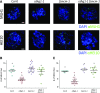
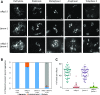
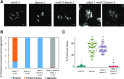
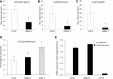

Comment in
-
Double Crossed: CDKG1 Regulates Crossover Formation by Stabilizing Meiotic and Somatic Recombination Intermediates.Plant Cell. 2020 Apr;32(4):814-815. doi: 10.1105/tpc.20.00162. Epub 2020 Feb 28. Plant Cell. 2020. PMID: 32111667 Free PMC article. No abstract available.
Similar articles
-
The Fanconi anemia ortholog FANCM ensures ordered homologous recombination in both somatic and meiotic cells in Arabidopsis.Plant Cell. 2012 Apr;24(4):1448-64. doi: 10.1105/tpc.112.096644. Epub 2012 Apr 30. Plant Cell. 2012. PMID: 22547783 Free PMC article.
-
CDKG1 protein kinase is essential for synapsis and male meiosis at high ambient temperature in Arabidopsis thaliana.Proc Natl Acad Sci U S A. 2014 Feb 11;111(6):2182-7. doi: 10.1073/pnas.1318460111. Epub 2014 Jan 27. Proc Natl Acad Sci U S A. 2014. PMID: 24469829 Free PMC article.
-
Zip4/Spo22 is required for class I CO formation but not for synapsis completion in Arabidopsis thaliana.PLoS Genet. 2007 May 25;3(5):e83. doi: 10.1371/journal.pgen.0030083. PLoS Genet. 2007. PMID: 17530928 Free PMC article.
-
ASY1 coordinates early events in the plant meiotic recombination pathway.Cytogenet Genome Res. 2008;120(3-4):302-12. doi: 10.1159/000121079. Epub 2008 May 23. Cytogenet Genome Res. 2008. PMID: 18504359 Review.
-
Meiotic chromosome synapsis and recombination in Arabidopsis thaliana: new ways of integrating cytological and molecular approaches.Chromosome Res. 2014 Jun;22(2):179-90. doi: 10.1007/s10577-014-9426-8. Chromosome Res. 2014. PMID: 24941912 Review.
Cited by
-
A Functional Kinase Is Necessary for Cyclin-Dependent Kinase G1 (CDKG1) to Maintain Fertility at High Ambient Temperature in Arabidopsis.Front Plant Sci. 2020 Nov 10;11:586870. doi: 10.3389/fpls.2020.586870. eCollection 2020. Front Plant Sci. 2020. PMID: 33240303 Free PMC article.
-
Double Crossed: CDKG1 Regulates Crossover Formation by Stabilizing Meiotic and Somatic Recombination Intermediates.Plant Cell. 2020 Apr;32(4):814-815. doi: 10.1105/tpc.20.00162. Epub 2020 Feb 28. Plant Cell. 2020. PMID: 32111667 Free PMC article. No abstract available.
-
Normal, novel or none: versatile regulation from alternative splicing.Plant Signal Behav. 2021 Jul 3;16(7):1917170. doi: 10.1080/15592324.2021.1917170. Epub 2021 Apr 22. Plant Signal Behav. 2021. PMID: 33882794 Free PMC article.
-
The UBP14-CDKB1;1-CDKG2 cascade controls endoreduplication and cell growth in Arabidopsis.Plant Cell. 2022 Mar 29;34(4):1308-1325. doi: 10.1093/plcell/koac002. Plant Cell. 2022. PMID: 34999895 Free PMC article.
-
Meiosis in allopolyploid Arabidopsis suecica.Plant J. 2022 Aug;111(4):1110-1122. doi: 10.1111/tpj.15879. Epub 2022 Jul 22. Plant J. 2022. PMID: 35759495 Free PMC article.
References
-
- Abdullah M.F.F., Hoffmann E.R., Cotton V.E., Borts R.H.(2004). A role for the MutL homologue MLH2 in controlling heteroduplex formation and in regulating between two different crossover pathways in budding yeast. Cytogenet. Genome Res. 107: 180–190. - PubMed
-
- Allers T., Lichten M.(2001). Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106: 47–57. - PubMed
-
- Armstrong S.J., Caryl A.P., Jones G.H., Franklin F.C.H.(2002). Asy1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brassica. J. Cell Sci. 115: 3645–3655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

