Meningeal Lymphangiogenesis and Enhanced Glymphatic Activity in Mice with Chronically Implanted EEG Electrodes
- PMID: 32047056
- PMCID: PMC7083292
- DOI: 10.1523/JNEUROSCI.2223-19.2020
Meningeal Lymphangiogenesis and Enhanced Glymphatic Activity in Mice with Chronically Implanted EEG Electrodes
Abstract
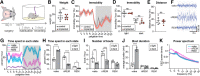
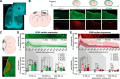
Chronic electroencephalography (EEG) is a widely used tool for monitoring cortical electrical activity in experimental animals. Although chronic implants allow for high-quality, long-term recordings in preclinical studies, the electrodes are foreign objects and might therefore be expected to induce a local inflammatory response. We here analyzed the effects of chronic cranial electrode implantation on glymphatic fluid transport and in provoking structural changes in the meninges and cerebral cortex of male and female mice. Immunohistochemical analysis of brain tissue and dura revealed reactive gliosis in the cortex underlying the electrodes and extensive meningeal lymphangiogenesis in the surrounding dura. Meningeal lymphangiogenesis was also evident in mice prepared with the commonly used chronic cranial window. Glymphatic influx of a CSF tracer was significantly enhanced at 30 d postsurgery in both awake and ketamine-xylazine anesthetized mice with electrodes, supporting the concept that glymphatic influx and intracranial lymphatic drainage are interconnected. Altogether, the experimental results provide clear evidence that chronic implantation of EEG electrodes is associated with significant changes in the brain's fluid transport system. Future studies involving EEG recordings and chronic cranial windows must consider the physiological consequences of cranial implants, which include glial scarring, meningeal lymphangiogenesis, and increased glymphatic activity.SIGNIFICANCE STATEMENT This study shows that implantation of extradural electrodes provokes meningeal lymphangiogenesis, enhanced glymphatic influx of CSF, and reactive gliosis. The analysis based on CSF tracer injection in combination with immunohistochemistry showed that chronically implanted electroencephalography electrodes were surrounded by lymphatic sprouts originating from lymphatic vasculature along the dural sinuses and the middle meningeal artery. Likewise, chronic cranial windows provoked lymphatic sprouting. Tracer influx assessed in coronal slices was increased in agreement with previous reports identifying a close association between glymphatic activity and the meningeal lymphatic vasculature. Lymphangiogenesis in the meninges and altered glymphatic fluid transport after electrode implantation have not previously been described and adds new insights to the foreign body response of the CNS.
Keywords: CSF; astrogliosis; dura; glial scarring; lymphatic; meninges.
Copyright © 2020 the authors.
Figures


Similar articles
-
Vasomotor influences on glymphatic-lymphatic coupling and solute trafficking in the central nervous system.J Cereb Blood Flow Metab. 2020 Aug;40(8):1724-1734. doi: 10.1177/0271678X19874134. Epub 2019 Sep 10. J Cereb Blood Flow Metab. 2020. PMID: 31506012 Free PMC article.
-
Transcranial direct current stimulation alters cerebrospinal fluid-interstitial fluid exchange in mouse brain.Brain Stimul. 2024 May-Jun;17(3):620-632. doi: 10.1016/j.brs.2024.04.009. Epub 2024 Apr 28. Brain Stimul. 2024. PMID: 38688399
-
Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage.Exp Neurol. 2019 Oct;320:113003. doi: 10.1016/j.expneurol.2019.113003. Epub 2019 Jun 28. Exp Neurol. 2019. PMID: 31260658 Free PMC article.
-
Current understanding of lymphatic vessels in the central nervous system.Neurosurg Rev. 2020 Aug;43(4):1055-1064. doi: 10.1007/s10143-019-01133-0. Epub 2019 Jun 18. Neurosurg Rev. 2020. PMID: 31209659 Review.
-
[Lymphatic system in central nervous system].Med Sci (Paris). 2019 Jan;35(1):55-61. doi: 10.1051/medsci/2018309. Epub 2019 Jan 23. Med Sci (Paris). 2019. PMID: 30672459 Review. French.
Cited by
-
Glymphatic system: a gateway for neuroinflammation.Neural Regen Res. 2024 Dec 1;19(12):2661-2672. doi: 10.4103/1673-5374.391312. Epub 2023 Dec 21. Neural Regen Res. 2024. PMID: 38595285 Free PMC article.
-
Fluid transport in the brain.Physiol Rev. 2022 Apr 1;102(2):1025-1151. doi: 10.1152/physrev.00031.2020. Epub 2021 May 5. Physiol Rev. 2022. PMID: 33949874 Free PMC article. Review.
-
Implantation of a Cranial Window for Repeated In Vivo Imaging in Awake Mice.J Vis Exp. 2021 Jun 22;(172):10.3791/62633. doi: 10.3791/62633. J Vis Exp. 2021. PMID: 34251372 Free PMC article.
-
Modeling lymphangiogenesis: Pairing in vitro and in vivo metrics.Microcirculation. 2023 Apr;30(2-3):e12802. doi: 10.1111/micc.12802. Epub 2023 Feb 28. Microcirculation. 2023. PMID: 36760223 Free PMC article. Review.
-
Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate.Nat Immunol. 2022 Apr;23(4):581-593. doi: 10.1038/s41590-022-01158-6. Epub 2022 Mar 28. Nat Immunol. 2022. PMID: 35347285 Free PMC article.
References
-
- Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, Fang S, Aspelund A, Saarma M, Eichmann A, Thomas JL, Alitalo K (2017) Development and plasticity of meningeal lymphatic vessels. J Exp Med 214:3645–3667. 10.1084/jem.20170391 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
