Drug resistance and tolerance in fungi
- PMID: 32047294
- PMCID: PMC7231573
- DOI: 10.1038/s41579-019-0322-2
Drug resistance and tolerance in fungi
Erratum in
-
Author Correction: Drug resistance and tolerance in fungi.Nat Rev Microbiol. 2020 Sep;18(9):539. doi: 10.1038/s41579-020-0415-y. Nat Rev Microbiol. 2020. PMID: 32601439
Abstract
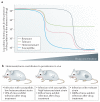
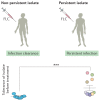
Systemic fungal infections pose a serious clinical problem. Treatment options are limited, and antifungal drug resistance is increasing. In addition, a substantial proportion of patients do not respond to therapy despite being infected with fungi that are susceptible to the drug. The discordance between overall treatment outcome and low levels of clinical resistance may be attributable to antifungal drug tolerance. In this Review, we define and distinguish resistance and tolerance and discuss the current understanding of the molecular, genetic and physiological mechanisms that contribute to those phenomena. Distinguishing tolerance from resistance might provide important insights into the reasons for treatment failure in some settings.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Andes DR, et al. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Transplant infectious disease : an official journal of the Transplantation Society. 2016;18:921–931. doi: 10.1111/tid.12613. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

