Variable in Vivo and in Vitro Biological Effects of Cerium Oxide Nanoparticle Formulations
- PMID: 32047435
- PMCID: PMC6997543
- DOI: 10.3389/fphar.2019.01599
Variable in Vivo and in Vitro Biological Effects of Cerium Oxide Nanoparticle Formulations
Abstract
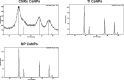
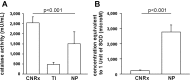
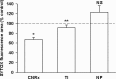
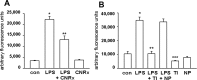
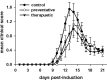
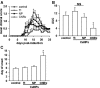
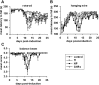
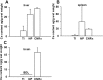
Cerium oxide nanoparticles (CeNPs) exhibit redox capacity in vitro with efficacy in in vivo disease models of oxidative stress. Here we compare, in parallel, three CeNP formulations with distinct chemical stabilizers and size. In vitro assays revealed antioxidant activity from all the CeNPs, but when administered to mice with a reactive oxygen species (ROS) mediated model of multiple sclerosis, only custom-synthesized Cerion NRx (CNRx) citrate-EDTA stabilized CeNPs provided protection against disease. Detectable levels of ceria and reduced ROS levels in the brains of CNRx CeNP-treated mice imply that these CeNPs' unique properties influence tissue distribution and subsequent biological activity, suggesting why differing CeNP formulations yield different in vivo effects in various models. Further, the variation in in vivo vs in vitro results with these CeNP formulations highlights the necessity for in vivo studies that confirm whether the inherent catalytic activity of CeNPs is maintained after transport and distribution within intact biological systems.
Keywords: antioxidants; cerium oxide nanoparticles; experimental autoimmune encephalomyelitis; macrophages; oxidative stress.
Copyright © 2020 Heckman, Estevez, DeCoteau, Vangellow, Ribeiro, Chiarenzelli, Hays-Erlichman and Erlichman.
Figures

References
LinkOut - more resources
Full Text Sources

