Recombinant Lactococcus lactis Expressing Ling Zhi 8 Protein Ameliorates Nonalcoholic Fatty Liver and Early Atherogenesis in Cholesterol-Fed Rabbits
- PMID: 32047809
- PMCID: PMC7007749
- DOI: 10.1155/2020/3495682
Recombinant Lactococcus lactis Expressing Ling Zhi 8 Protein Ameliorates Nonalcoholic Fatty Liver and Early Atherogenesis in Cholesterol-Fed Rabbits
Abstract
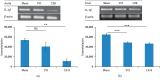
Atherosclerosis is an inflammatory disease characterized by lipid deposits in the subendothelial space leading to severe inflammation. Nonalcoholic fatty liver disease (NAFLD) shares several risk factors with atherosclerosis, including dyslipidemia, type 2 diabetes mellitus, and metabolic syndrome, all of which lead to lipid deposition in the liver causing inflammation and fibrosis. Several clinical trials have shown that certain Chinese herbal medicines with anti-inflammatory effects can be used as adjuvant therapy to prevent the development of cardiovascular events and liver disease. Ling Zhi 8 (LZ8) is an immunomodulatory protein isolated from a medicinal mushroom and has been well documented to possess a broad range of pharmacological properties. This study aimed to evaluate the protective effects of recombinant Lactococcus lactis expressing LZ8 protein on NAFLD and atherogenesis in a cholesterol-fed rabbit model. Twelve rabbits were divided into three groups and fed with syrup only, L. lactis vehicle, or recombinant L. lactis-LZ8 once a day on weekdays for five weeks, respectively. The gene expression of IL-1β in the aorta was significantly suppressed after oral administration of L. lactis-LZ8. Moreover, in hematoxylin and eosin staining of the aorta, the intima-medial thickness was decreased, and foam cells were significantly reduced in the subendothelial space. LZ8 also inhibited the expression of IL-1β in the liver, decreased fat droplet deposits and infiltration of inflammatory cells, and improved liver function by decreasing liver enzymes in an animal model. Our results suggest that the Lactococcus-expressing LZ8 appears to be a promising medicine for improving both NAFLD and early atherogenesis owing to its anti-inflammatory effect. Furthermore, it is available as a low-cost food-grade product.
Copyright © 2020 Mey-Fann Lee et al.
Conflict of interest statement
The authors have no conflicts of interest.
Figures




References
-
- Athyros V. G., Alexandrides T. K., Bilianou H., et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An expert panel statement. Metabolism. 2017;71:17–32. doi: 10.1016/j.metabol.2017.02.014. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

