Immune profile differences between chronic GVHD and late acute GVHD: results of the ABLE/PBMTC 1202 studies
- PMID: 32047896
- PMCID: PMC7146024
- DOI: 10.1182/blood.2019003186
Immune profile differences between chronic GVHD and late acute GVHD: results of the ABLE/PBMTC 1202 studies
Abstract
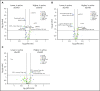
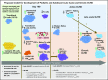
Human graft-versus-host disease (GVHD) biology beyond 3 months after hematopoietic stem cell transplantation (HSCT) is complex. The Applied Biomarker in Late Effects of Childhood Cancer study (ABLE/PBMTC1202, NCT02067832) evaluated the immune profiles in chronic GVHD (cGVHD) and late acute GVHD (L-aGVHD). Peripheral blood immune cell and plasma markers were analyzed at day 100 post-HSCT and correlated with GVHD diagnosed according to the National Institutes of Health consensus criteria (NIH-CC) for cGVHD. Of 302 children enrolled, 241 were evaluable as L-aGVHD, cGVHD, active L-aGVHD or cGVHD, and no cGVHD/L-aGVHD. Significant marker differences, adjusted for major clinical factors, were defined as meeting all 3 criteria: receiver-operating characteristic area under the curve ≥0.60, P ≤ .05, and effect ratio ≥1.3 or ≤0.75. Patients with only distinctive features but determined as cGVHD by the adjudication committee (non-NIH-CC) had immune profiles similar to NIH-CC. Both cGVHD and L-aGVHD had decreased transitional B cells and increased cytolytic natural killer (NK) cells. cGVHD had additional abnormalities, with increased activated T cells, naive helper T (Th) and cytotoxic T cells, loss of CD56bright regulatory NK cells, and increased ST2 and soluble CD13. Active L-aGVHD before day 114 had additional abnormalities in naive Th, naive regulatory T (Treg) cell populations, and cytokines, and active cGVHD had an increase in PD-1- and a decrease in PD-1+ memory Treg cells. Unsupervised analysis appeared to show a progression of immune abnormalities from no cGVHD/L-aGVHD to L-aGVHD, with the most complex pattern in cGVHD. Comprehensive immune profiling will allow us to better understand how to minimize L-aGVHD and cGVHD. Further confirmation in adult and pediatric cohorts is needed.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
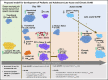
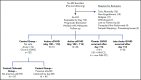
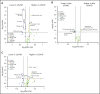
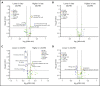

Comment in
-
A few steps on the long road toward biomarkers in GVHD.Blood. 2020 Apr 9;135(15):1196-1197. doi: 10.1182/blood.2020005116. Blood. 2020. PMID: 32271904 No abstract available.
References
-
- Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12(2):126-137. - PubMed
-
- She K, Gilman AL, Aslanian S, et al. Altered Toll-like receptor 9 responses in circulating B cells at the onset of pediatric chronic GVHD. Biol Blood Marrow Transplant. 2007;13:386-397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

