Metabolism, ATP production and biofilm generation by Staphylococcus epidermidis in either respiratory or fermentative conditions
- PMID: 32048056
- PMCID: PMC7013028
- DOI: 10.1186/s13568-020-00966-z
Metabolism, ATP production and biofilm generation by Staphylococcus epidermidis in either respiratory or fermentative conditions
Abstract
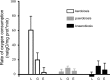
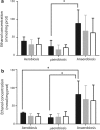
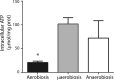
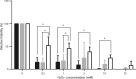
Staphylococcus epidermidis is a Gram-positive saprophytic bacterium found in the microaerobic/anaerobic layers of the skin that becomes a health hazard when it is carried across the skin through punctures or wounds. Pathogenicity is enhanced by the ability of S. epidermidis to associate into biofilms, where it avoids attacks by the host and antibiotics. To test the effect of oxygen on metabolism and biofilm generation, cells were cultured at different oxygen concentrations ([O2]). As [O2] decreased, S. epidermidis metabolism went from respiratory to fermentative. Remarkably, the rate of growth decreased at low [O2] while a high concentration of ATP ([ATP]) was kept. Under hypoxic conditions bacteria associated into biofilms. Aerobic activity sensitized the cell to hydrogen peroxide-mediated damage. In the presence of metabolic inhibitors, biofilm formation decreased. It is suggested that at low [O2] S. epidermidis limits its growth and develops the ability to form biofilms.
Keywords: Biofilms; Fermentation; Metabolism; Oxygen concentration; Rate of oxygen consumption; Staphylococcus epidermidis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Staphylococcus epidermidis: metabolic adaptation and biofilm formation in response to different oxygen concentrations.Pathog Dis. 2016 Feb;74(1):ftv111. doi: 10.1093/femspd/ftv111. Epub 2015 Nov 25. Pathog Dis. 2016. PMID: 26610708
-
Effect of incubation atmosphere on the production and composition of staphylococcal biofilms.J Infect Chemother. 2015 Jan;21(1):55-61. doi: 10.1016/j.jiac.2014.10.001. Epub 2014 Oct 31. J Infect Chemother. 2015. PMID: 25454214
-
PhoU2 but Not PhoU1 as an Important Regulator of Biofilm Formation and Tolerance to Multiple Stresses by Participating in Various Fundamental Metabolic Processes in Staphylococcus epidermidis.J Bacteriol. 2017 Nov 14;199(24):e00219-17. doi: 10.1128/JB.00219-17. Print 2017 Dec 15. J Bacteriol. 2017. PMID: 28947672 Free PMC article.
-
Antibiofilm activity of three Actinomycete strains against Staphylococcus epidermidis.Lett Appl Microbiol. 2019 Jan;68(1):73-80. doi: 10.1111/lam.13087. Epub 2018 Nov 22. Lett Appl Microbiol. 2019. PMID: 30338533 Review.
-
Structural basis of Staphylococcus epidermidis biofilm formation: mechanisms and molecular interactions.Front Cell Infect Microbiol. 2015 Feb 17;5:14. doi: 10.3389/fcimb.2015.00014. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25741476 Free PMC article. Review.
Cited by
-
Staphylococcus epidermidis biofilms undergo metabolic and matrix remodeling under nitrosative stress.Front Cell Infect Microbiol. 2023 Jul 4;13:1200923. doi: 10.3389/fcimb.2023.1200923. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37469594 Free PMC article.
-
In vitro evaluation of microbial D- and L-lactate production as biomarkers of infection.Front Microbiol. 2024 Aug 8;15:1406350. doi: 10.3389/fmicb.2024.1406350. eCollection 2024. Front Microbiol. 2024. PMID: 39176282 Free PMC article.
-
Bacterial extracellular vesicle: A non-negligible component in biofilm life cycle and challenges in biofilm treatments.Biofilm. 2024 Jul 25;8:100216. doi: 10.1016/j.bioflm.2024.100216. eCollection 2024 Dec. Biofilm. 2024. PMID: 39184814 Free PMC article. Review.
-
Local erythropoiesis directs oxygen availability in bone fracture repair.bioRxiv [Preprint]. 2025 Jan 11:2025.01.10.632440. doi: 10.1101/2025.01.10.632440. bioRxiv. 2025. PMID: 39829797 Free PMC article. Preprint.
-
Bio-based polylactic acid labware as a sustainable alternative for microbial cultivation in life science laboratories.Heliyon. 2024 Oct 26;10(21):e39846. doi: 10.1016/j.heliyon.2024.e39846. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39539974 Free PMC article.
References
-
- Beck S, Sehl C, Voortmann S, Verhasselt HL, Edwards MJ, Buer J, Hasenberg M, Gulbins E, Becker KA. Sphingosine is able to prevent and eliminate Staphylococcus epidermidis biofilm formation on different orthopedic implant materials in vitro. J Mol Med. 2019 doi: 10.1007/s00109-019-01858-x. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

