Deletion of Protein Kinase D3 Promotes Liver Fibrosis in Mice
- PMID: 32048304
- PMCID: PMC9338785
- DOI: 10.1002/hep.31176
Deletion of Protein Kinase D3 Promotes Liver Fibrosis in Mice
Abstract
Background and aims: Liver fibrosis (LF) is a central pathological process that occurs in most types of chronic liver diseases. Advanced LF causes cirrhosis, hepatocellular carcinoma, and liver failure. However, the exact molecular mechanisms underlying the initiation and progression of LF remain largely unknown.
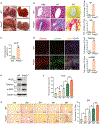
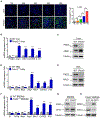
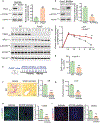
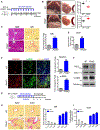
Approach and results: This study was designed to investigate the role of protein kinase D3 (PKD3; gene name Prkd3) in the regulation of liver homeostasis. We generated global Prkd3 knockout (Prkd3-/- ) mice and myeloid-cell-specific Prkd3 knockout (Prkd3∆LysM ) mice, and we found that both Prkd3-/- mice and Prkd3∆LysM mice displayed spontaneous LF. PKD3 deficiency also aggravated CCl4 -induced LF. PKD3 is highly expressed in hepatic macrophages (HMs), and PKD3 deficiency skewed macrophage polarization toward a profibrotic phenotype. Activated profibrotic macrophages produced transforming growth factor beta that, in turn, activates hepatic stellate cells to become matrix-producing myofibroblasts. Moreover, PKD3 deficiency decreased the phosphatase activity of SH2-containing protein tyrosine phosphatase-1 (a bona-fide PKD3 substrate), resulting in sustained signal transducer and activator of transcription 6 activation in macrophages. In addition, we observed that PKD3 expression in HMs was down-regulated in cirrhotic human liver tissues.
Conclusions: PKD3 deletion in mice drives LF through the profibrotic macrophage activation.
© 2020 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures



References
-
- Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol 2018;68–69:435–451. - PubMed
-
- Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol 2018;68:238–250. - PubMed
-
- Karsdal MA, Hjuler ST, Luo Y, Rasmussen DGK, Nielsen MJ, Holm Nielsen S, et al. Assessment of liver fibrosis progression and regression by a serological collagen turnover profile. Am J Physiol Gastrointest Liver Physiol 2019;316:G25–G31. - PubMed
-
- Vollmar J, Kim YO, Marquardt JU, Becker D, Galle PR, Schuppan D, et al. Deletion of organic cation transporter Oct3 promotes hepatic fibrosis via upregulation of TGFβ. Am J Physiol Gastrointest Liver Physiol 2019;317:G195–G202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

