Automated, Accelerated Nanoscale Synthesis of Iminopyrrolidines
- PMID: 32048418
- PMCID: PMC7383484
- DOI: 10.1002/anie.202000887
Automated, Accelerated Nanoscale Synthesis of Iminopyrrolidines
Abstract
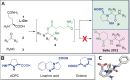
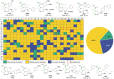
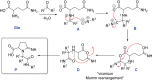
Miniaturization and acceleration of synthetic chemistry is an emerging area in pharmaceutical, agrochemical, and materials research and development. Herein, we describe the synthesis of iminopyrrolidine-2-carboxylic acid derivatives using chiral glutamine, oxo components, and isocyanide building blocks in an unprecedented Ugi-3-component reaction. We used I-DOT, a positive-pressure-based low-volume and non-contact dispensing technology to prepare more than 1000 different derivatives in a fully automated fashion. In general, the reaction is stereoselective, proceeds in good yields, and tolerates a wide variety of functional groups. We exemplify a pipeline of fast and efficient nanomole-scale scouting to millimole-scale synthesis for the discovery of a useful novel reaction with great scope.
Keywords: automation; miniaturization; multicomponent reactions; nanoscale synthesis; sustainable chemistry.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mattes D. S., Jung N., Weber L. K., Bräse S., Breitling F., Adv. Mater. 2019, 31, 1806656. - PubMed
-
- None
-
- Uehling M. R., King R. P., Krska S. W., Cernak T., Buchwald S. L., Science 2019, 363, 405; - PubMed
-
- Lin S., Dikler S., Blincoe W. D., Ferguson R. D., Sheridan R. P., Peng Z., Conway D. V., Zawatzky K., Wang H., Cernak T., Davies I. W., DiRocco D. A., Sheng H., Welch C. J., Dreher S. D., Science 2018, 361, eaar6236; - PubMed
-
- Gesmundo N. J., Sauvagnat B., Curran P. J., Richards M. P., Andrews C. L., Dandliker P. J., Cernak T., Nature 2018, 557, 228–232; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

