Derivation of trophoblast stem cells from naïve human pluripotent stem cells
- PMID: 32048992
- PMCID: PMC7062471
- DOI: 10.7554/eLife.52504
Derivation of trophoblast stem cells from naïve human pluripotent stem cells
Abstract
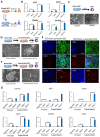
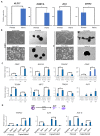
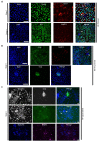
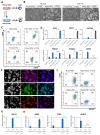
Naïve human pluripotent stem cells (hPSCs) provide a unique experimental platform of cell fate decisions during pre-implantation development, but their lineage potential remains incompletely characterized. As naïve hPSCs share transcriptional and epigenomic signatures with trophoblast cells, it has been proposed that the naïve state may have enhanced predisposition for differentiation along this extraembryonic lineage. Here we examined the trophoblast potential of isogenic naïve and primed hPSCs. We found that naïve hPSCs can directly give rise to human trophoblast stem cells (hTSCs) and undergo further differentiation into both extravillous and syncytiotrophoblast. In contrast, primed hPSCs do not support hTSC derivation, but give rise to non-self-renewing cytotrophoblasts in response to BMP4. Global transcriptome and chromatin accessibility analyses indicate that hTSCs derived from naïve hPSCs are similar to blastocyst-derived hTSCs and acquire features of post-implantation trophectoderm. The derivation of hTSCs from naïve hPSCs will enable elucidation of early mechanisms that govern normal human trophoblast development and associated pathologies.
Keywords: ATAC-seq; RNA-seq; human; naive pluripotency; primed pluripotency; regenerative medicine; stem cells; trophectoderm; trophoblast stem cells.
Plain language summary
The placenta is one of the most important human organs, but it is perhaps the least understood. The first decision the earliest human cells have to make, shortly after the egg is fertilized by a sperm, is whether to become part of the embryo or part of the placenta. This choice happens before a pregnancy even implants into the uterus. The cells that commit to becoming the embryo transform into ‘naïve pluripotent’ cells, capable of becoming any cell in the body. Those that commit to becoming the placenta transform into ‘trophectoderm’ cells, capable of becoming the two types of cell in the placenta. Placental cells either invade into the uterus to anchor the placenta or produce hormones to support the pregnancy. Once a pregnancy implants into the uterus, the naïve pluripotent cells in the embryo become ‘primed’. This prevents them from becoming cells of the placenta, and it poses a problem for placental research. In 2018, scientists in Japan reported conditions for growing trophectoderm cells in the laboratory, where they are known as “trophoblast stem cells”. These cells were capable of transforming into specialized placental cells, but needed first to be isolated from the human embryo or placenta itself. Dong et al. now show how to reprogram other pluripotent cells grown in the laboratory to produce trophoblast stem cells. The first step was to reset primed pluripotent cells to put them back into a naïve state. Then, Dong et al. exposed the cells to the same concoction of nutrients and chemicals used in the 2018 study. This fluid triggered a transformation in the naïve pluripotent cells; they started to look like trophoblast stem cells, and they switched on genes normally active in trophectoderm cells. To test whether these cells had the same properties as trophoblast stem cells, Dong et al. gave them chemical signals to see if they could mature into placental cells. The stem cells were able to transform into both types of placental cell, either invading through a three-dimensional gel that mimics the wall of the uterus or making pregnancy hormones. There is a real need for a renewable supply of placental cells in pregnancy research. Animal placentas are not the same as human ones, so it is not possible to learn everything about human pregnancy from animal models. A renewable supply of trophoblast stem cells could aid in studying how the placenta forms and why this process sometimes goes wrong. This could help researchers to better understand miscarriage, pre-eclampsia and other conditions that affect the growth of an unborn baby. In the future, it may even be possible to make custom trophoblast stem cells to study the specific fertility issues of an individual.
© 2020, Dong et al.
Conflict of interest statement
CD, MB, PG, BZ, PP, LF, SK, KP, EY, XX, RK, TW, TT No competing interests declared, LS Reviewing editor, eLife
Figures
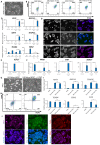
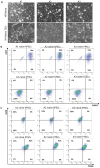
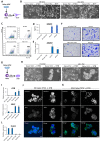
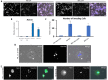


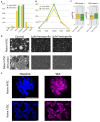
References
-
- Allison TF, Andrews PW, Avior Y, Barbaric I, Benvenisty N, Bock C, Brehm J, Brüstle O, Damjanov I, Elefanty A, International Stem Cell Initiative Assessment of established techniques to determine developmental and malignant potential of human pluripotent stem cells. Nature Communications. 2018;9:1925. doi: 10.1038/s41467-018-04011-3. - DOI - PMC - PubMed
-
- Bernardo AS, Faial T, Gardner L, Niakan KK, Ortmann D, Senner CE, Callery EM, Trotter MW, Hemberger M, Smith JC, Bardwell L, Moffett A, Pedersen RA. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

