Assessment of Clinical Activity of PD-1 Checkpoint Inhibitor Combination Therapies Reported in Clinical Trials
- PMID: 32049290
- PMCID: PMC12549068
- DOI: 10.1001/jamanetworkopen.2019.20833
Assessment of Clinical Activity of PD-1 Checkpoint Inhibitor Combination Therapies Reported in Clinical Trials
Abstract
Importance: Because cancer drugs given in combination have the potential for increased tumor-cell killing, finding the best combination partners for programmed cell death 1 (PD-1) checkpoint inhibitors could improve clinical outcomes for patients with cancer.
Objective: To identify optimal strategies for combining PD-1 immune checkpoint inhibitors with other cancer therapies.
Design, setting, and participants: This cross-sectional study compiled 319 results from 98 clinical trials testing PD-1 pathway inhibitors alone or in combination with other agents among 24 915 patients with metastatic cancer. All clinical trials had a primary completion date before September 16, 2018. Data analysis was conducted from November 2018 to August 2019.
Exposures: Patients with metastatic cancer were treated with PD-1 immune checkpoint inhibitors alone or with other cancer therapies.
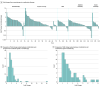
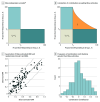
Main outcomes and measures: Clinical activity was measured as objective response rates (ORRs). Combination measures included fold change from monotherapy to combination ORR, comparison of observed combination ORRs with estimated combination ORRs based on independent additivity, and a computational model to assess clinical synergy. To assess whether the ORRs of various combinations may be greater than the independent contribution of each agent, a Bliss independent activity model was used to analyze observed combination ORRs, and a Z score, measuring the difference between observed and calculated ORRs, was generated.
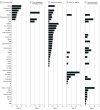
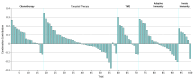
Results: In 319 results from 98 clinical trials among 24 915 patients, ORRs for monotherapy were compared with combination data by indication and line of therapy, demonstrating an increased ORR in 105 of 127 results (82.7%) where ORRs were available for both PD-1 pathway inhibitor monotherapy and combination therapy. A few combinations showed increases above the Bliss-estimated activity, possibly identifying limited clinical synergy. The mean (SD) Z score for all trials was 0.0430 (0.0243). The mean (SD) Z score was 0.0923 (0.0628) for platinum chemotherapy regimen combinations, 0.0547 (0.0821) for vascular endothelial growth factor or vascular endothelial growth factor receptor tyrosine kinase inhibitor combinations, 0.0893 (0.086) for indoleamine 2,3-dioxygenase inhibitor combinations, and 0.0558 (0.0849) for cytotoxic T-lymphocyte-associated protein 4 inhibitor combinations.
Conclusions and relevance: In this cross-sectional study, most combination trials showed the expected benefit of combining 2 active anticancer agents, but few combination trials showed clinical synergy according to the Bliss independent activity model.
Conflict of interest statement
Figures
References
-
- Law LW. Effects of combinations of antileukemic agents on an acute lymphocytic leukemia of mice. Cancer Res. 1952;12(12):871-878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

