Comparison of Tightly Controlled Dose Reduction of Biologics With Usual Care for Patients With Psoriasis: A Randomized Clinical Trial
- PMID: 32049319
- PMCID: PMC7042801
- DOI: 10.1001/jamadermatol.2019.4897
Comparison of Tightly Controlled Dose Reduction of Biologics With Usual Care for Patients With Psoriasis: A Randomized Clinical Trial
Abstract
Importance: Biologics revolutionized the treatment of psoriasis. Biologics are given in a fixed dose, but lower doses might be possible.
Objective: To investigate whether dose reduction (DR) of biologics in patients with stable psoriasis is noninferior to usual care (UC).
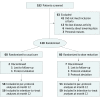
Design, setting, and participants: This pragmatic, open-label, prospective, controlled, noninferiority randomized clinical trial was conducted from March 1, 2016, to July 22, 2018, at 6 dermatology departments in the Netherlands. A total of 120 patients with plaque psoriasis and stable low disease activity who were receiving treatment with adalimumab, etanercept, or ustekinumab were studied.
Interventions: Patients were randomized 1:1 to DR (n = 60) or UC (n = 60). In the DR group, injection intervals were prolonged stepwise, leading to 67% and 50% of the original dose.
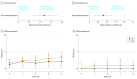
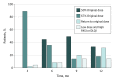
Main outcomes and measures: The primary outcome was between-group difference in disease activity corrected for baseline at 12 months compared with the predefined noninferiority margin of 0.5. Secondary outcomes were Psoriasis Area and Severity Index (PASI) score and health-related quality of life (including Dermatology Life Quality Index [DLQI] and Medical Outcomes Study 36-Item Short Form Health Survey scores), proportion of patients with short and persistent flares (defined as PASI and/or DLQI scores >5 for ≥3 months), and proportion of patients with successful dose tapering.
Results: Of 120 patients (mean [SD] age, 54.0 [13.2] years; 82 [68%] male), 2 patients were lost to follow-up, 2 patients had a protocol violation, and 5 patients had a protocol deviation, leaving 111 patients for the per-protocol analysis (53 in the DR group and 58 in the UC group). The median PASI scores at month 12 were 3.4 (interquartile range [IQR], 2.2-4.5) in the DR group and 2.1 (IQR, 0.6-3.6) in the UC group (mean difference, 1.2; 95% CI, 0.7-1.8). This indicates that noninferiority was not demonstrated for DR compared to UC. The median DLQI score at month 12 was 1.0 (IQR, 0.0-2.0) in the DR group and 0.0 (IQR, 0.0-2.0) in the UC group (mean difference, 0.8; 95% CI, 0.3-1.3), indicating noninferiority for DR compared with UC. No significant difference was found regarding persistent flares between groups (n = 5 in both groups). Twenty-eight patients (53%; 95% CI, 39%-67%) in the DR group tapered their dose successfully at 12 months. No severe adverse events related to the intervention occurred.
Conclusions and relevance: In this trial, noninferiority was not demonstrated for DR of adalimumab, etanercept, and ustekinumab based on the PASI in patients with psoriasis compared with UC with the chosen noninferiority margin. However, the strategy was noninferior based on the DLQI. Dose tapering did not lead to persistent flares or safety issues.
Trial registration: ClinicalTrials.gov Identifier: NCT02602925.
Conflict of interest statement
Figures
References
-
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275 - DOI - PubMed
-
- Papp K, Menter A, Poulin Y, Gu Y, Sasso EH. Long-term outcomes of interruption and retreatment vs. continuous therapy with adalimumab for psoriasis: subanalysis of REVEAL and the open-label extension study. J Eur Acad Dermatol Venereol. 2013;27(5):634-642. doi:10.1111/j.1468-3083.2012.04515.x - DOI - PubMed
-
- Gordon KB, Gottlieb AB, Langely RG, et al. . Adalimumab retreatment successfully restores clinical response and health-related quality of life in patients with moderate to severe psoriasis who undergo therapy interruption. J Eur Acad Dermatol Venereol. 2015;29(4):767-776. doi:10.1111/jdv.12677 - DOI - PubMed

