The Glutathione/Metallothionein System Challenges the Design of Efficient O2 -Activating Copper Complexes
- PMID: 32049413
- PMCID: PMC7294961
- DOI: 10.1002/anie.201916316
The Glutathione/Metallothionein System Challenges the Design of Efficient O2 -Activating Copper Complexes
Abstract
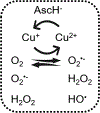
Copper complexes are of medicinal and biological interest, including as anticancer drugs designed to cleave intracellular biomolecules by O2 activation. To exhibit such activity, the copper complex must be redox active and resistant to dissociation. Metallothioneins (MTs) and glutathione (GSH) are abundant in the cytosol and nucleus. Because they are thiol-rich reducing molecules with high CuI affinity, they are potential competitors for a copper ion bound in a copper drug. Herein, we report the investigation of a panel of CuI /CuII complexes often used as drugs, with diverse coordination chemistries and redox potentials. We evaluated their catalytic activity in ascorbate oxidation based on redox cycling between CuI and CuII , as well as their resistance to dissociation or inactivation under cytosolically relevant concentrations of GSH and MT. O2 -activating CuI /CuII complexes for cytosolic/nuclear targets are generally not stable against the GSH/MT system, which creates a challenge for their future design.
Keywords: bioinorganic chemistry; copper-based drugs; glutathione; metallothionein; redox activity.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

