Electrochemical Oxidation of Organic Molecules at Lower Overpotential: Accessing Broader Functional Group Compatibility with Electron-Proton Transfer Mediators
- PMID: 32049487
- PMCID: PMC7295176
- DOI: 10.1021/acs.accounts.9b00544
Electrochemical Oxidation of Organic Molecules at Lower Overpotential: Accessing Broader Functional Group Compatibility with Electron-Proton Transfer Mediators
Abstract
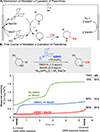
Electrochemical organic oxidation reactions are highly appealing because protons are often effective terminal electron acceptors, thereby avoiding undesirable stoichiometric oxidants. These reactions are often plagued by high overpotentials, however, that greatly limit their utility. Single-electron transfer (SET) from organic molecules generates high-energy radical-cations. Formation of such intermediates often requires electrode potentials far above the thermodynamic potentials of the reaction and frequently causes decomposition and/or side reactions of ancillary functional groups. In this Account, we show how electrocatalytic electron-proton transfer mediators (EPTMs) address this challenge. EPTMs bypass the formation of radical-cation intermediates by supporting mechanisms that operate at electrode potentials much lower (≥1 V) than those of analogous direct electrolysis reactions.The stable aminoxyl radical TEMPO (2,2,6,6-tetramethylpiperidine N-oxyl) is an effective mediator for electrochemical alcohol oxidation, and we have employed such processes for applications ranging from pharmaceutical synthesis to biomass conversion. A complementary electrochemical alcohol oxidation method employs a cooperative Cu/TEMPO mediator system that operates at 0.5 V lower electrode potential than the TEMPO-only mediated process. This difference, which arises from a different catalytic mechanism, rationalizes the broad functional group tolerance of Cu/TEMPO-based aerobic alcohol oxidation catalysts.Aminoxyl mediators address long-standing challenges in the "Shono oxidation," an important method for α-C-H oxidation of tertiary amides and carbamates. Shono oxidations are initiated by a high-potential SET step that limits their utility. Aminoxyl-mediated Shono-type oxidations have been developed that operate at much lower potentials and tolerate diverse functional groups. Analogous reactivity underlies α-C-H cyanation of secondary cyclic amines, a new method that enables efficient diversification of piperidine-based pharmaceutical building blocks and preparation of non-natural amino acids.Electrochemical oxidations of benzylic C-H bonds are commonly initiated by SET to generate arene radical cations, but such methods are again plagued by large overpotentials. Mediated electrolysis methods that promote hydrogen-atom-transfer (HAT) from benzylic C-H bonds to Fe-oxo species and phthalimide N-oxyl (PINO) support C-H oxygenation, iodination, and oxidative-coupling reactions. A complementary method merges photochemistry with electrochemistry to achieve amidation of C(sp3)-H bonds. This unique process operates at much lower overpotentials compatible with diverse functional groups.These results have broad implications for organic electrochemistry, highlighting the importance of "overpotential" considerations and the prospects for expanding synthetic utility by using mediators to bypass high-energy outer-sphere electron-transfer mechanisms. Principles demonstrated here for oxidation are equally relevant to electrochemical reductions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
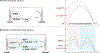



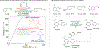












References
-
- Caron S; Dugger RW; Ruggeri SG; Ragan JA; Ripin DHB, Large-Scale Oxidations in the Pharmaceutical Industry. Chem. Rev. 2006, 106, 2943–2989. - PubMed
-
- Modern Oxidation Methods, 2nd Ed.; Bäckvall J-E, Ed.; Wiley-VCH: Weinheim, Germany, 2010.
-
- Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives; Stahl SS, Alsters PL, Eds.; Wiley-VCH: Weinheim, Germany, 2016.
-
- Green Oxidation in Organic Synthesis; Jiao N; Stahl SS, Eds.; Wiley: Hoboken, NJ, 2019.
-
- Frontana-Uribe BA; Little RD; Ibanez JG; Palma A; Vasquez-Medrano R Organic Electrosynthesis: A Promising Green Methodology in Organic Chemistry. Green Chem. 2010, 12, 2099–2119.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

