Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: a phase II, randomized, double-blind, placebo-controlled trial
- PMID: 32049923
- PMCID: PMC7188038
- DOI: 10.1097/GME.0000000000001497
Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: a phase II, randomized, double-blind, placebo-controlled trial
Abstract
Objective: To assess the efficacy and safety of ultra-low dose 0.005% estriol vaginal gel in women with breast cancer receiving nonsteroidal aromatase inhibitors (NSAIs) and experiencing treatment-related vulvovaginal symptoms and signs.
Methods: Women with hormone receptor-positive early breast cancer receiving NSAIs were randomized to either estriol vaginal gel or placebo for 12 weeks. Vaginal maturation, vaginal pH, and total and individual scores of symptoms and signs of vulvovaginal atrophy were assessed at baseline and at weeks 3 and 12; sexual functioning was also evaluated using the Female Sexual Functioning Index (FSFI) questionnaire, as well as circulating estrogens, follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
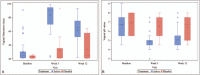
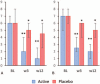
Results: Sixty-one women with a mean age of 59 years were included: 50 received 0.005% estriol vaginal gel and 11 received placebo. Active treatment significantly improved maturation value and pH, vaginal dryness and global scores of symptoms and signs. Active treatment also increased the total FSFI score and all the FSFI domains, with the exception of pain. Small oscillations were observed in FSH and LH, which remained within the postmenopausal range. Estriol levels increased initially and normalized by week 12, and estradiol and estrone remained mostly undetectable throughout the study.
Conclusions: Ultra-low dose 0.005% estriol vaginal gel showed efficacy in improving the symptoms and signs of vulvovaginal atrophy. These results, together with minimal oscillations in hormonal levels throughout the treatment, support the use of ultra-low dose 0.005% estriol vaginal gel as a treatment option for vulvovaginal atrophy in women with breast cancer receiving NSAIs with an indication for treatment with vaginal estrogens. : Video Summary:http://links.lww.com/MENO/A531.
Plain language summary
Video Summary:http://links.lww.com/MENO/A531.
Conflict of interest statement
Financial disclosure/conflicts of interest: PS-R has participated in educational activities sponsored by Roche, Kern-Pharma, Pfizer, Novartis, and Astra Zeneca; has received research support from Roche, BMS, and Merck; fees as consultant or advisory board member from Roche, Pfizer, Novartis, and Daiichi; and grants for attending conferences from Roche, Kern-Pharma, and Pfizer. MG-G has received fees as consultant or advisory board member from Pfizer, Novartis, Roche, and Eisai, and grants for attending conferences from Pfizer and Roche. AL-H has received a grant from ITF Research Pharma for attending a conference. M C-D, EL, and JP-L report no conflicts of interest. JS and CN are full time employees of ITF Research Pharma.
Figures


References
-
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068. - PubMed
-
- Hickey M, Saunders C, Partridge A, et al. Practical clinical guidelines for assessing and managing menopausal symptoms after breast cancer. Ann Oncol 2008; 19:1669–1680. - PubMed
-
- Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J Clin Oncol 1998; 16:501–514. - PubMed

