A high infectious simian adenovirus type 23 vector based vaccine efficiently protects common marmosets against Zika virus infection
- PMID: 32049958
- PMCID: PMC7015313
- DOI: 10.1371/journal.pntd.0008027
A high infectious simian adenovirus type 23 vector based vaccine efficiently protects common marmosets against Zika virus infection
Abstract
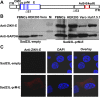
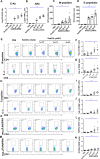
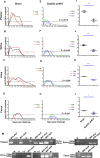
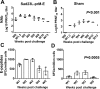
Zika virus (ZIKV) has spread in many countries or territories causing severe neurologic complications with potential fatal outcomes. The small primate common marmosets are susceptible to ZIKV, mimicking key features of human infection. Here, a novel simian adenovirus type 23 vector-based vaccine expressing ZIKV pre-membrane-envelope proteins (Sad23L-prM-E) was produced in high infectious titer. Due to determination of immunogenicity in mice, a single-dose of 3×108 PFU Sad23L-prM-E vaccine was intramuscularly inoculated to marmosets. This vaccine raised antibody titers of 104.07 E-specific and 103.13 neutralizing antibody (NAb), as well as robust specific IFN-γ secreting T-cell response (1,219 SFCs/106 cells) to E peptides. The vaccinated marmosets, upon challenge with a high dose of ZIKV (105 PFU) six weeks post prime immunization, reduced viremia by more than 100 folds, and the low level of detectable viral RNA (<103 copies/ml) in blood, saliva, urine and feces was promptly eliminated when the secondary NAb (titer >103.66) and T-cell response (>726 SFCs/106 PBMCs) were acquired 1-2 weeks post exposure to ZIKV, while non-vaccinated control marmosets developed long-term high titer of ZIKV (105.73 copies/ml) (P<0.05). No significant pathological lesions were observed in marmoset tissues. Sad23L-prM-E vaccine was detectable in spleen, liver and PBMCs at least 4 months post challenge. In conclusion, a prime immunization with Sad23L-prM-E vaccine was able to protect marmosets against ZIKV infection when exposed to a high dose of ZIKV. This Sad23L-prM-E vaccine is a promising vaccine candidate for prevention of ZIKV infection in humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
A rapid strategy for constructing novel simian adenovirus vectors with high viral titer and expressing highly antigenic proteins applicable for vaccine development.Virus Res. 2019 Jul 15;268:1-10. doi: 10.1016/j.virusres.2019.05.008. Epub 2019 May 17. Virus Res. 2019. PMID: 31108113
-
Recombinant Chimpanzee Adenovirus Vaccine AdC7-M/E Protects against Zika Virus Infection and Testis Damage.J Virol. 2018 Feb 26;92(6):e01722-17. doi: 10.1128/JVI.01722-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298885 Free PMC article.
-
Immunization With a Novel Human Type 5 Adenovirus-Vectored Vaccine Expressing the Premembrane and Envelope Proteins of Zika Virus Provides Consistent and Sterilizing Protection in Multiple Immunocompetent and Immunocompromised Animal Models.J Infect Dis. 2018 Jul 2;218(3):365-377. doi: 10.1093/infdis/jiy187. J Infect Dis. 2018. PMID: 29617816
-
Zika virus structural biology and progress in vaccine development.Biotechnol Adv. 2018 Jan-Feb;36(1):47-53. doi: 10.1016/j.biotechadv.2017.09.004. Epub 2017 Sep 12. Biotechnol Adv. 2018. PMID: 28916391 Review.
-
Potential targets for therapeutic intervention and structure based vaccine design against Zika virus.Eur J Med Chem. 2018 Aug 5;156:444-460. doi: 10.1016/j.ejmech.2018.07.014. Epub 2018 Jul 10. Eur J Med Chem. 2018. PMID: 30015077 Review.
Cited by
-
Two Novel Adenovirus Vectors Mediated Differential Antibody Responses via Interferon-α and Natural Killer Cells.Microbiol Spectr. 2023 Aug 17;11(4):e0088023. doi: 10.1128/spectrum.00880-23. Epub 2023 Jun 22. Microbiol Spectr. 2023. PMID: 37347197 Free PMC article.
-
Exploring T-Cell Immunity to Hepatitis C Virus: Insights from Different Vaccine and Antigen Presentation Strategies.Vaccines (Basel). 2024 Aug 6;12(8):890. doi: 10.3390/vaccines12080890. Vaccines (Basel). 2024. PMID: 39204016 Free PMC article. Review.
-
Track-etched membrane microplate and smartphone immunosensing for SARS-CoV-2 neutralizing antibody.Biosens Bioelectron. 2021 Nov 15;192:113550. doi: 10.1016/j.bios.2021.113550. Epub 2021 Aug 8. Biosens Bioelectron. 2021. PMID: 34391066 Free PMC article.
-
Prime-boost vaccination of mice and rhesus macaques with two novel adenovirus vectored COVID-19 vaccine candidates.Emerg Microbes Infect. 2021 Dec;10(1):1002-1015. doi: 10.1080/22221751.2021.1931466. Emerg Microbes Infect. 2021. PMID: 33993845 Free PMC article.
-
A nanoenzyme linked immunochromatographic sensor for rapid and quantitative detection of SARS-CoV-2 nucleocapsid protein in human blood.Sens Actuators B Chem. 2021 Dec 15;349:130718. doi: 10.1016/j.snb.2021.130718. Epub 2021 Sep 12. Sens Actuators B Chem. 2021. PMID: 34539081 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

