Pre-treatment EMG can be used to model post-treatment muscle coordination during walking in children with cerebral palsy
- PMID: 32050002
- PMCID: PMC7015404
- DOI: 10.1371/journal.pone.0228851
Pre-treatment EMG can be used to model post-treatment muscle coordination during walking in children with cerebral palsy
Abstract
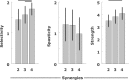
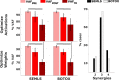
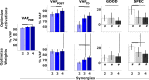
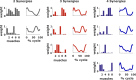
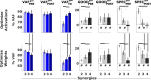
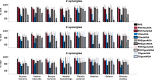
When treating children with Cerebral Palsy (CP), computational simulations based on musculoskeletal models have a great potential in assisting the clinical decision-making process towards the most promising treatments. In particular, predictive simulations could be used to predict and compare the functional outcome of a series of candidate interventions. In order to be able to benefit from these predictive simulations however, it is important to know how much information about the post-treatment patient's motor control could be gathered from data available before the intervention. Within this paper, we quantified how much of the muscle activity measured after a treatment could be explained by subject-specific muscle synergies computed from EMG data collected before the intervention. We also investigated whether generic synergies could be used, in case no EMG data is available when running predictive simulations, to reproduce both pre- and post-treatment muscle activity in children with CP. Subject-specific synergies proved to be a good indicator of the patient's post-treatment motor control, explaining on average more than 85% of the post-treatment muscle activity, compared to an average of 94% when applied to the original pre-treatment data. Generic synergies explained 84% of the pre-treatment and 83% of the post-treatment muscle activity on average, but performed relatively well for patients with low selective motor control and poorly in patients with more selectivity. Our results suggest that subject-specific muscle synergies computed from pre-treatment EMG data could be used with confidence to represent the post-treatment motor control of children with CP during walking. In addition, when performing simulations involving patients with a low selective motor control, generic synergies could be a valid alternative.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

