RASS-Enabled S/P-C and S-N Bond Formation for DEL Synthesis
- PMID: 32050046
- PMCID: PMC7486030
- DOI: 10.1002/anie.201915493
RASS-Enabled S/P-C and S-N Bond Formation for DEL Synthesis
Abstract
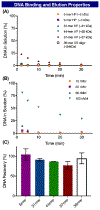
DNA encoded libraries (DEL) have shown promise as a valuable technology for democratizing the hit discovery process. Although DEL provides relatively inexpensive access to libraries of unprecedented size, their production has been hampered by the idiosyncratic needs of the encoding DNA tag relegating DEL compatible chemistry to dilute aqueous environments. Recently reversible adsorption to solid support (RASS) has been demonstrated as a promising method to expand DEL reactivity using standard organic synthesis protocols. Here we demonstrate a suite of on-DNA chemistries to incorporate medicinally relevant and C-S, C-P and N-S linkages into DELs, which are underrepresented in the canonical methods.
Keywords: DNA encoded library; nickel cross-coupling; reversible adsorption to solid support; sulfonamide; sulfone.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Kowalczyk A, A Handbook for DNA-Encoded Chemistry: Theory and Applications for Exploring Chemical Space and Drug Discovery (Ed.: Goodnow RE) Wiley-VCH: Weinheim, Germany, 2014, pp. 1–18.
-
- Belyanskaya SL, Ding Y, Callahan JF, Lazaar AL and Israel DI, ChemBioChem 2017, 18, 837–842. - PubMed
-
- Lerner RA and Brenner S, Angew. Chem. Int. Ed 2017, 56, 1164–1165. - PubMed
-
- Mannocci L, Leimbacher M, Wichert M, Scheuermann J and Neri D, Chem. Commun 2011, 47, 12747–12753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

