Visible-Light-Enabled Direct Decarboxylative N-Alkylation
- PMID: 32050048
- PMCID: PMC7200280
- DOI: 10.1002/anie.201916710
Visible-Light-Enabled Direct Decarboxylative N-Alkylation
Abstract
The development of efficient and selective C-N bond-forming reactions from abundant feedstock chemicals remains a central theme in organic chemistry owing to the key roles of amines in synthesis, drug discovery, and materials science. Herein, we present a dual catalytic system for the N-alkylation of diverse aromatic carbocyclic and heterocyclic amines directly with carboxylic acids, by-passing their preactivation as redox-active esters. The reaction, which is enabled by visible-light-driven, acridine-catalyzed decarboxylation, provides access to N-alkylated secondary and tertiary anilines and N-heterocycles. Additional examples, including double alkylation, the installation of metabolically robust deuterated methyl groups, and tandem ring formation, further demonstrate the potential of the direct decarboxylative alkylation (DDA) reaction.
Keywords: amination; carboxylic acids; copper catalysis; photocatalysis; visible light.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




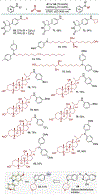
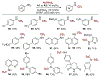

References
-
- Lawrence SA, Amines: Synthesis Properties and Applications, Cambridge University Press, Cambridge, 2004;
- Travis AS in The Chemistry of Anilines, Parts 1 & 2, (Ed.: Rappoport Z), Wiley, Chichester, 2007, pp 715–782.
- Ricci A, Amino Group Chemistry: From Synthesis to the Life Sciences, Wiley-VCH, Weinheim, 2008.
-
- Qiao JX, Lam PYS in Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. (Ed.: Hall DG), Wiley-VCH, Weinheim, 2011, pp. 315–361;
- Lam PYS, in Synthetic Methods in Drug Discovery, Vol 1, (Eds.: Blakemore D, Doyle P, Fobian Y), The Royal Society of Chemistry, 2016, pp. 242–273.
-
- Gomez S, Peters JA, Maschmeyer T, Adv. Synth. Catal 2002, 344, 1037–1057;
- Shi F, Cui X, Catalytic Amination for N-Alkyl Amine Synthesis, Academic Press, London, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

