The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules
- PMID: 32050446
- PMCID: PMC7037960
- DOI: 10.3390/molecules25030745
The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules
Abstract
During 2019, the US Food and Drug Administration (FDA) approved 48 new drugs (38 New Chemical Entities and 10 Biologics). Although this figure is slightly lower than that registered in 2018 (59 divided between 42 New Chemical Entities and 17 Biologics), a year that broke a record with respect to new drugs approved by this agency, it builds on the trend initiated in 2017, when 46 drugs were approved. Of note, three antibody drug conjugates, three peptides, and two oligonucleotides were approved in 2019. This report analyzes the 48 new drugs of the class of 2019 from a strictly chemical perspective. The classification, which was carried out on the basis of chemical structure, includes the following: Biologics (antibody drug conjugates, antibodies, and proteins); TIDES (peptide and oligonucleotides); drug combinations; natural products; and small molecules.
Keywords: API; TIDES; antibodies; antibody drug conjugate; biologics; chemical entities; drug discovery; fluorine-based drugs; natural products; oligonucleotides; peptides; pyrazoles; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

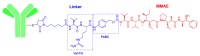
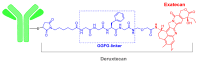
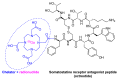



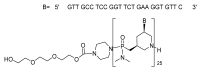
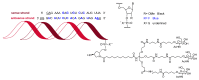





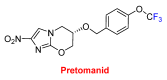
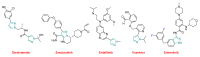

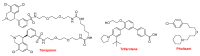

References
-
- U.S. Food and Drug Administration (FDA) [(accessed on 24 January 2020)]; Available online: http://www.accessdata.fda.gov/scripts/cder/daf/
-
- Jarvis L.M. The new drugs of 2018. Chem. Eng. News. 2019;97:33–37.
-
- Jarvis L.M. The new drugs of 2019. Chem. Eng. News. 2020;98:30–36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

