Aristolochic Acid-Induced Nephrotoxicity: Molecular Mechanisms and Potential Protective Approaches
- PMID: 32050524
- PMCID: PMC7043226
- DOI: 10.3390/ijms21031157
Aristolochic Acid-Induced Nephrotoxicity: Molecular Mechanisms and Potential Protective Approaches
Abstract
Aristolochic acid (AA) is a generic term that describes a group of structurally related compounds found in the Aristolochiaceae plants family. These plants have been used for decades to treat various diseases. However, the consumption of products derived from plants containing AA has been associated with the development of nephropathy and carcinoma, mainly the upper urothelial carcinoma (UUC). AA has been identified as the causative agent of these pathologies. Several studies on mechanisms of action of AA nephrotoxicity have been conducted, but the comprehensive mechanisms of AA-induced nephrotoxicity and carcinogenesis have not yet fully been elucidated, and therapeutic measures are therefore limited. This review aimed to summarize the molecular mechanisms underlying AA-induced nephrotoxicity with an emphasis on its enzymatic bioactivation, and to discuss some agents and their modes of action to reduce AA nephrotoxicity. By addressing these two aspects, including mechanisms of action of AA nephrotoxicity and protective approaches against the latter, and especially by covering the whole range of these protective agents, this review provides an overview on AA nephrotoxicity. It also reports new knowledge on mechanisms of AA-mediated nephrotoxicity recently published in the literature and provides suggestions for future studies.
Keywords: DNA adducts formation; aristolochic acid; aristolochic acid nephrotoxicity; potential protective mechanisms; upper urothelial carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
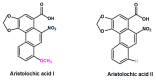


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

