Extracellular Vesicles Mediated Early Embryo-Maternal Interactions
- PMID: 32050564
- PMCID: PMC7037557
- DOI: 10.3390/ijms21031163
Extracellular Vesicles Mediated Early Embryo-Maternal Interactions
Abstract
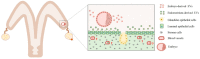
Embryo-maternal crosstalk is an important event that involves many biological processes, which must occur perfectly for pregnancy success. This complex communication starts from the zygote stage within the oviduct and continues in the uterus up to the end of pregnancy. Small extracellular vesicles (EVs) are part of this communication and carry bioactive molecules such as proteins, lipids, mRNA, and miRNA. Small EVs are present in the oviductal and uterine fluid and have important functions during fertilization and early embryonic development. Embryonic cells are able to uptake oviductal and endometrium-derived small EVs. Conversely, embryo-derived EVs might modulate oviductal and uterine function. In this review, our aim is to demonstrate the role of extracellular vesicles modulating embryo-maternal interactions during early pregnancy.
Keywords: blood circulating exosomes; crosstalk; embryo; endometrium; oviduct; small extracellular vesicles.
Conflict of interest statement
The authors declared no conflict of interest.
Figures
References
-
- Betteridge K.J., Fléchon J.E. The anatomy and physiology of pre-attachment bovine embryos. Theriogenology. 1988;29:155–187. doi: 10.1016/0093-691X(88)90038-6. - DOI
Publication types
MeSH terms
Grants and funding
- 2014/22887-0/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2018/13155-6/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2017/19681-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 306349/2017-5/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 420152/2018-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico
LinkOut - more resources
Full Text Sources

