Coupling of Cell Division and Differentiation in Arabidopsis thaliana Cultured Cells with Interaction of Ethylene and ABA Signaling Pathways
- PMID: 32050697
- PMCID: PMC7175341
- DOI: 10.3390/life10020015
Coupling of Cell Division and Differentiation in Arabidopsis thaliana Cultured Cells with Interaction of Ethylene and ABA Signaling Pathways
Abstract
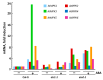
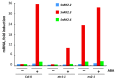
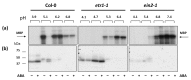
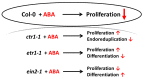
Recent studies indicate direct links between molecular cell cycle and cell differentiation machineries. Ethylene and abscisic acid (ABA) are known to affect cell division and differentiation, but the mechanisms of such effects are poorly understood. As ethylene and ABA signaling routes may interact, we examined their involvement in cell division and differentiation in cell tissue cultures derived from several Arabidopsis thaliana plants: wild type (Col-0), and ethylene-insensitive mutants etr1-1, ctr1-1, and ein2-1. We designed an experimental setup to analyze the growth-related parameters and molecular mechanisms in proliferating cells upon short exposure to ABA. Here, we provide evidence for the ethylene-ABA signaling pathways' interaction in the regulation of cell division and differentiation as follows: (1) when the ethylene signal transduction pathway is functionally active (Col-0), the cells actively proliferate, and exogenous ABA performs its function as an inhibitor of DNA synthesis and division; (2) if the ethylene signal is not perceived (etr1-1), then, in addition to cell differentiation (tracheary elements formation), cell death can occur. The addition of exogenous ABA can rescue the cells via increasing proliferation; (3) if the ethylene signal is perceived, but not transduced (ein2-1), then cell differentiation takes place-the latter is enhanced by exogenous ABA while cell proliferation is reduced; (4) when the signal transduction pathway is constitutively active, the cells begin to exit the cell cycle and proceed to endo-reduplication (ctr1-1). In this case, the addition of exogenous ABA promotes reactivation of cell division.
Keywords: Arabidopsis thaliana; abscisic acid; cell culture; cell differentiation; cell proliferation; ethylene.
Conflict of interest statement
The authors declare no conflict of interest. The funders played no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Dudits D., Abraham E., Miskolczi P., Ayaydin F., Bilgin M., Gabor V., Horvath G.V. Cell-cycle control as a target for calcium, hormonal and developmental signals: the role of phosphorylation in the retinoblastoma-centered pathway. Ann. Bot. 2005;107:193–1202. doi: 10.1093/aob/mcr038. - DOI - PMC - PubMed
-
- Ruggiero B., Koiwa H., Manabe Y., Quist T.M., Inan G., Saccardo F., Joly R.J., Hasegawa P.M., Bressan R.A., Maggio A. Uncoupling the effects of abscisic acid on plant growth and water relations. Analysis of sto1/nced3, an abscisic acid-deficient but salt stress-tolerant mutant in Arabidopsis. Plant Physiol. 2004;136:3134–3147. doi: 10.1104/pp.104.046169. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

