Implementation of the Goal-directed Medication review Electronic Decision Support System (G-MEDSS)© into home medicines review: a protocol for a cluster-randomised clinical trial in older adults
- PMID: 32050899
- PMCID: PMC7017507
- DOI: 10.1186/s12877-020-1442-2
Implementation of the Goal-directed Medication review Electronic Decision Support System (G-MEDSS)© into home medicines review: a protocol for a cluster-randomised clinical trial in older adults
Erratum in
-
Correction to: Implementation of the goal-directed medication review electronic decision support system (G-MEDSS)© into home medicines review: a protocol for a clusterrandomised clinical trial in older adults.BMC Geriatr. 2020 Oct 2;20(1):378. doi: 10.1186/s12877-020-01681-x. BMC Geriatr. 2020. PMID: 33008288 Free PMC article.
Abstract
Background: Older people living in the community have a high prevalence of polypharmacy and are vulnerable to adverse drug events. Home Medicines Review (HMR) is a collaborative medication review service involving general practitioners (GPs), accredited clinical pharmacists (ACPs) and patients, which aims to prevent medication-related problems. This study aims to evaluate the implementation of a Computerised Clinical Decision Support System (CCDSS) called G-MEDSS© (Goal-directed Medication Review Electronic Decision Support System) in HMRs to deprescribe anticholinergic and sedative medications, and to assess the effect of deprescribing on clinical outcomes.
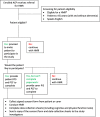
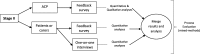
Methods: This study consists of 2 stages: Stage I - a two-arm parallel-group cluster-randomised clinical trial, and Stage II - process evaluation of the CCDSS intervention in HMR. Community-dwelling older adults living with and without dementia who are referred for HMR by their GP and recruited by ACPs will be included in this study. G-MEDSS is a CCDSS designed to provide clinical decision support for healthcare practitioners when completing a medication review, to tailor care to meet the patients' goals and preferences. The G-MEDSS contains three tools: The Goals of Care Management Tool, The Drug Burden Index (DBI) Calculator©, and The revised Patients' Attitudes Towards Deprescribing (rPATD) questionnaire. The G-MEDSS produces patient-specific deprescribing reports, to be included as part of the ACPs communication with the patient's GP, and patient-specific reports for the patient (or carer). ACPs randomised to the intervention arm of the study will use G-MEDSS to create deprescribing reports for the referring GP and for their patient (or carer) when submitting the HMR report. ACPs in the comparison arm will provide the usual care HMR service (without the G-MEDSS).
Outcomes: The primary outcome is reduction in DBI exposure 3 months after HMR ± G-MEDSS intervention between comparison and intervention groups. The secondary outcomes include changes in clinical outcomes (physical and cognitive function, falls, institutionalisation, GP visits, medication adherence and mortality) 3-months after HMR.
Discussion: This study is expected to add to the evidence that the combination of CCDSS supporting medication review can improve prescribing and clinical outcomes in older adults.
Trial registration: The trial was registered on the Australian New Zealand Clinical Trials Registry ACTRN12617000895381 on 19th June 2017.
Keywords: Dementia; Deprescribing; Drug burden index; Home medicines review; Older adults; Patient centred care.
Conflict of interest statement
The authors declare that they have no competing interests.
The Goal directed Medication review Electronic Decision Support System (G-MEDSS)© Copyright 2019. The University of Sydney. All Rights Reserved. The Drug Burden Index (DBI) Calculator© Copyright 2019. The University of Sydney. All rights reserved.
Figures


References
-
- Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Cumming RG, Handelsman DJ, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther. 2012;91(3):521–528. - PubMed
-
- Morgan TK, Williamson M, Pirotta M, Stewart K, Myers SP, Barnes J. A national census of medicines use: a 24-hour snapshot of Australians aged 50 years and older. Med J Aust. 2012;196(1):50–53. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

