Comparison of retention in observational cohorts and nested simulated HIV vaccine efficacy trials in the key populations in Uganda
- PMID: 32050900
- PMCID: PMC7014936
- DOI: 10.1186/s12874-020-00920-4
Comparison of retention in observational cohorts and nested simulated HIV vaccine efficacy trials in the key populations in Uganda
Abstract
Background: Outcomes in observational studies may not best estimate those expected in the HIV vaccine efficacy trials. We compared retention in Simulated HIV Vaccine Efficacy Trials (SiVETs) and observational cohorts drawn from two key populations in Uganda.
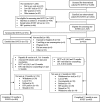
Methods: Two SiVETs were nested within two observational cohorts, one in Fisherfolk (FF) and another one in Female Sex Workers (FSW). Adult participants in each observational cohort were screened for enrolment into SiVETs. Those screened-out or not screened continued participation in the observational (non-SiVET) cohorts. SiVET participants were administered a licensed hepatitis B vaccine in a schedule that mimicked an actual HIV vaccine efficacy trial. Both cohorts were followed for 12 months and retention was assessed through dropout, defined as lost to follow up, being uncontactable, refusal to continue or missing the last study clinic visit. Dropout rates were compared using Poisson models giving rate ratios and 95% confidence intervals (95%CI).
Results: Out of 1525 participants (565 FF and 960 FSW), 572 (38%) were enrolled into SiVETs (282-FF and 290-FSW), and 953 (62%) remained in the non-SiVET cohorts. Overall, 326 (101 SiVET, 225 non-SiVET) dropped out in 1260 Person Years of Observation (PYO), a dropout rate of 25.9 /100 PYO (95%CI: 23.2-28.8); fewer dropped out in the SiVET cohorts (18.4, 95% CI: 15.1-22.4) than in the non-SiVET cohorts (31.6, 95% CI: 27.8-36.1), rate ratio (RR) =0.58, 95% CI: 0.46-0.73. In all cohorts, the dropout was more marked in FSW than in FF population. Duration lived in community was associated with dropout in both SiVETs and religion in both non-SiVET cohorts.
Conclusion: The rate of dropout was lower in SiVET compared to non-SiVET cohort. Though the difference in dropout between SiVET and non-SiVET was generally similar, the actual dropout rates were higher in the FSW population. Conduct of SiVETs in these key populations could mean that designing HIV Vaccine Efficacy Trials will benefit from lower dropout rate shown in SiVET than non-SiVET observational cohort.
Keywords: Retention dropout observational cohorts vaccine trials key-populations.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Predictors of Loss to Follow-Up in an HIV Vaccine Preparedness Study in Masaka, Uganda.Int J Environ Res Public Health. 2022 May 24;19(11):6377. doi: 10.3390/ijerph19116377. Int J Environ Res Public Health. 2022. PMID: 35681962 Free PMC article. Review.
-
Simulated vaccine efficacy trials to estimate HIV incidence for actual vaccine clinical trials in key populations in Uganda.Vaccine. 2019 Apr 3;37(15):2065-2072. doi: 10.1016/j.vaccine.2019.02.072. Epub 2019 Mar 8. Vaccine. 2019. PMID: 30857933
-
Use of propensity score matching to create counterfactual group to assess potential HIV prevention interventions.Sci Rep. 2021 Mar 29;11(1):7017. doi: 10.1038/s41598-021-86539-x. Sci Rep. 2021. PMID: 33782485 Free PMC article.
-
Comparison of HIV Risk Behaviors Between Clinical Trials and Observational Cohorts in Uganda.AIDS Behav. 2020 Oct;24(10):2872-2884. doi: 10.1007/s10461-020-02838-w. AIDS Behav. 2020. PMID: 32277309 Free PMC article.
-
Effect of race/ethnicity on participation in HIV vaccine trials and comparison to other trials of biomedical prevention.Hum Vaccin Immunother. 2014;10(7):1974-84. doi: 10.4161/hv.28870. Hum Vaccin Immunother. 2014. PMID: 25424807 Free PMC article. Review.
Cited by
-
Feasibility of conducting HIV prevention trials among key populations in Nairobi, Kenya.BMC Public Health. 2022 Dec 20;22(1):2385. doi: 10.1186/s12889-022-14875-2. BMC Public Health. 2022. PMID: 36536335 Free PMC article.
-
Feasibility and acceptability of using biometric fingerprinting to track migrations and support retention in HIV prevention research in fishing population in East Africa.BMC Public Health. 2023 Dec 1;23(1):2383. doi: 10.1186/s12889-023-17339-3. BMC Public Health. 2023. PMID: 38041047 Free PMC article.
-
Predictors of Loss to Follow-Up in an HIV Vaccine Preparedness Study in Masaka, Uganda.Int J Environ Res Public Health. 2022 May 24;19(11):6377. doi: 10.3390/ijerph19116377. Int J Environ Res Public Health. 2022. PMID: 35681962 Free PMC article. Review.
References
-
- Development ICOAA. Understanding the research process for new HIV prevention technologies. 2010.
-
- UAC . Uganda national strategic plan for HIV&AIDS 2007/8–2011/12. 2007.
-
- Platt L, Grenfell P, Fletcher A, Sorhaindo A, Jolley E, Rhodes T, et al. Systematic review examining differences in HIV, sexually transmitted infections and health-related harms between migrant and non-migrant female sex workers. Sex Transm Infect. 2013;89(4):311–319. doi: 10.1136/sextrans-2012-050491. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

