Tumour necrosis factor alpha promotes secretion of 14-3-3η by inducing necroptosis in macrophages
- PMID: 32051018
- PMCID: PMC7017620
- DOI: 10.1186/s13075-020-2110-9
Tumour necrosis factor alpha promotes secretion of 14-3-3η by inducing necroptosis in macrophages
Abstract
Background: 14-3-3η is an intracellular protein also detected in the serum and synovial fluid of patients with rheumatoid arthritis (RA). It is closely related to disease activity and anti-cyclic citrullinated peptide antibody levels. However, the main source of 14-3-3η and the mechanism of its release into the extracellular space remain unclear. Addressing these two points was the main goal of the current study.
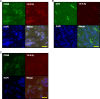
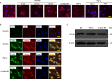
Methods: The source of 14-3-3η was investigated by immunostaining RA synovial tissue. Fibroblast-like synoviocytes, CD4+ cells, and macrophages were selected as candidates among the various cell types in the synovial tissue. Phosphorylation of mixed-lineage kinase domain-like pseudokinase (MLKL) and cell death of macrophages were studied by phalloidin staining and electron microscopy after stimulation with an oxidative stress inducer (diamide) or tumour necrosis factor (TNF)-α. Extracellular 14-3-3η protein levels were examined by western blotting.
Results: Macrophages from the synovial tissue from RA, but not osteoarthritis, showed dense and widespread cytoplasmic staining for the 14-3-3η protein, co-localized with peptidylarginine deiminase 4. Swelling and membrane rupture of macrophages were induced by treatment with TNF-α, but not interleukin (IL) 6/soluble IL-6 receptor (sIL-6R). Increased MLKL phosphorylation followed by necroptosis was also induced in TNF-α-stimulated macrophages. Necrostatin-1, a necroptosis inhibitor, antagonized MLKL phosphorylation. High levels of 14-3-3η were detected in the culture supernatants of macrophages stimulated with diamide and TNF-α, but not IL-6/sIL-6R.
Conclusions: Macrophages that highly express 14-3-3η undergo TNF-α-induced necroptosis with damage to the cellular structure, resulting in the secretion of 14-3-3η into the extracellular space. The current study provides a novel mechanism for 14-3-3η level increase in the RA synovial fluid.
Keywords: 14-3-3η; Macrophage; Necroptosis; Rheumatoid arthritis; TNF-α.
Conflict of interest statement
YT has received consulting fees, speaking fees, and/or honoraria from Daiichi-Sankyo, Astellas, Pfizer, Mitsubishi-Tanabe, Bristol-Myers, Chugai, YL Biologics, Eli Lilly, Sanofi, Janssen, and UCB, and has received research grants from Mitsubishi-Tanabe, Takeda, Bristol-Myers, Chugai, Astellas, Abbvie, MSD, Daiichi-Sankyo, Pfizer, Kyowa-Kirin, Eisai, and Ono. SN has received speaking fees from Bristol-Myers, UCB, Astellas, Abbvie, Eisai, Pfizer, and Takeda, and has received research grants from Mitsubishi-Tanabe, Novartis, and MSD. KY has received research grants from Mitsubishi-Tanabe. SH has received speaking fee and honoraria from AbbVie, Asahi-Kasei Pharma, Astellas, Ayumi, Bristol-Myers Squibb, Celgene, Chugai, Eisai, Eli Lilly, Janssen, Kissei, Novartis, Pfizer, Sanofi, Takeda, Tanabe-Mitsubishi, and UCB. NB is an employee of Augurex. All other authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

