Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages
- PMID: 32051167
- PMCID: PMC9488537
- DOI: 10.1183/16000617.0052-2019
Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages
Abstract
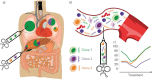
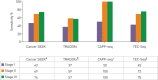
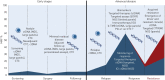
Liquid biopsy refers to the analysis of any tumour-derived material circulating in the blood or any other body fluid. This concept is particularly relevant in lung cancer as the tumour is often difficult to reach and may need an invasive and potentially harmful procedure. Moreover, the multitude of anticancer drugs and their sequential use underline the importance of conducting an iterative assessment of tumour biology. Liquid biopsies can noninvasively detect any targetable genomic alteration and guide corresponding targeted therapy, in addition to monitoring response to treatment and exploring the genetic changes at resistance, overcoming spatial and temporal heterogeneity.In this article, we review the available data in the field, which suggest the potential of liquid biopsy in the area of lung cancer, with a particular focus on cell-free DNA and circulating tumour cells. We discuss their respective applications in patient selection and monitoring through targeted therapy, as well as immune checkpoint inhibitors. The current data and future applications of liquid biopsy in the early stage setting are also investigated.Liquid biopsy has the potential to help manage nonsmall cell lung cancer throughout all stages of lung cancer: screening, minimal residual disease detection to guide adjuvant treatment, early detection of relapse, systemic treatment initiation and monitoring of response (targeted or immune therapy), and resistance genotyping.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: N. Guibert reports non-financial support from BMS, MSD and Pfizer, and personal fees from AstraZeneca, BMS, and MSD, outside the submitted work. Conflict of interest: A. Pradines has nothing to disclose. Conflict of interest: G. Favre has nothing to disclose. Conflict of interest: J. Mazieres has nothing to disclose.
Figures
References
-
- Best MG, Wesseling P, Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res 2018; 78: 3407–3412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
