Immunomodulatory Strategies in Herpes Simplex Virus Encephalitis
- PMID: 32051176
- PMCID: PMC7018500
- DOI: 10.1128/CMR.00105-19
Immunomodulatory Strategies in Herpes Simplex Virus Encephalitis
Abstract
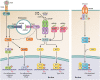
Herpes simplex virus 1 (HSV-1) can be responsible for life-threatening HSV encephalitis (HSE). The mortality rate of patients with HSE who do not receive antiviral treatment is 70%, with most survivors suffering from permanent neurological sequelae. The use of intravenous acyclovir together with improved diagnostic technologies such as PCR and magnetic resonance imaging has resulted in a reduction in the mortality rate to close to 20%. However, 70% of surviving patients still do not recover complete neurological functions. Thus, there is an urgent need to develop more effective treatments for a better clinical outcome. It is well recognized that cerebral damage resulting from HSE is caused by viral replication together with an overzealous inflammatory response. Both of these processes constitute potential targets for the development of innovative therapies against HSE. In this review, we discuss recent progress in therapy that may be used to ameliorate the outcome of patients with HSE, with a particular emphasis on immunomodulatory agents. Ideally, the administration of adjunctive immunomodulatory drugs should be initiated during the rise of the inflammatory response, and its duration should be limited in time to reduce undesired effects. This critical time frame should be optimized by the identification of reliable biomarkers of inflammation.
Keywords: encephalitis; herpes simplex virus; immune response; immunomodulatory drugs.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Baltimore, MD.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

