Thermally activated triplet exciton release for highly efficient tri-mode organic afterglow
- PMID: 32051404
- PMCID: PMC7016145
- DOI: 10.1038/s41467-020-14669-3
Thermally activated triplet exciton release for highly efficient tri-mode organic afterglow
Abstract
Developing high-efficient afterglow from metal-free organic molecules remains a formidable challenge due to the intrinsically spin-forbidden phosphorescence emission nature of organic afterglow, and only a few examples exhibit afterglow efficiency over 10%. Here, we demonstrate that the organic afterglow can be enhanced dramatically by thermally activated processes to release the excitons on the stabilized triplet state (T1*) to the lowest triplet state (T1) and to the singlet excited state (S1) for spin-allowed emission. Designed in a twisted donor-acceptor architecture with small singlet-triplet splitting energy and shallow exciton trapping depth, the thermally activated organic afterglow shows an efficiency up to 45%. This afterglow is an extraordinary tri-mode emission at room temperature from the radiative decays of S1, T1, and T1*. With the highest afterglow efficiency reported so far, the tri-mode afterglow represents an important concept advance in designing high-efficient organic afterglow materials through facilitating thermally activated release of stabilized triplet excitons.
Conflict of interest statement
The authors declare no competing interests.
Figures
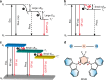
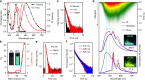



Similar articles
-
Modulating Tri-Mode Emission for Single-Component White Organic Afterglow.Angew Chem Int Ed Engl. 2021 Nov 15;60(47):24984-24990. doi: 10.1002/anie.202109229. Epub 2021 Oct 13. Angew Chem Int Ed Engl. 2021. PMID: 34523785
-
Fluorine-induced aggregate-interlocking for color-tunable organic afterglow with a simultaneously improved efficiency and lifetime.Chem Sci. 2021 Jan 12;12(10):3580-3586. doi: 10.1039/d0sc06025a. Chem Sci. 2021. PMID: 34163631 Free PMC article.
-
Constructing Organic Phosphorescent Scintillators with Enhanced Triplet Exciton Utilization Through Multi-Mode Radioluminescence for Efficient X-Ray Imaging.Adv Mater. 2024 Nov;36(46):e2409338. doi: 10.1002/adma.202409338. Epub 2024 Sep 23. Adv Mater. 2024. PMID: 39308317
-
Recent Advances in Thermally Activated Delayed Fluorescence-Based Organic Afterglow Materials.Small Methods. 2025 Mar;9(3):e2400982. doi: 10.1002/smtd.202400982. Epub 2024 Oct 25. Small Methods. 2025. PMID: 39460397 Review.
-
TADF Material Design: Photophysical Background and Case Studies Focusing on CuI and AgI Complexes.Chemphyschem. 2017 Dec 15;18(24):3508-3535. doi: 10.1002/cphc.201700872. Epub 2017 Dec 19. Chemphyschem. 2017. PMID: 29083512 Review.
Cited by
-
Tunable Photoinduced Charge Transfer at the Interface between Benzoselenadiazole-Based MOF Linkers and Thermally Activated Delayed Fluorescence Chromophore.J Phys Chem B. 2023 Mar 2;127(8):1819-1827. doi: 10.1021/acs.jpcb.2c08844. Epub 2023 Feb 21. J Phys Chem B. 2023. PMID: 36807993 Free PMC article.
-
On-demand modulating afterglow color of water-soluble polymers through phosphorescence FRET for multicolor security printing.Sci Adv. 2022 Apr 15;8(15):eabk2925. doi: 10.1126/sciadv.abk2925. Epub 2022 Apr 15. Sci Adv. 2022. PMID: 35427159 Free PMC article.
-
Organic Room Temperature Phosphorescence from BN-Substituted Xanthene Derivatives.Angew Chem Int Ed Engl. 2025 Jan 10;64(2):e202414534. doi: 10.1002/anie.202414534. Epub 2024 Nov 11. Angew Chem Int Ed Engl. 2025. PMID: 39406686 Free PMC article.
-
Triggering anti-Kasha organic room temperature phosphorescence of clusteroluminescent materials.Chem Sci. 2025 Mar 24;16(18):7829-7837. doi: 10.1039/d5sc01471a. eCollection 2025 May 7. Chem Sci. 2025. PMID: 40177316 Free PMC article.
-
Brightening triplet excitons enable high-performance white-light emission in organic small molecules via integrating n-π*/π-π* transitions.Nat Commun. 2024 Sep 5;15(1):7778. doi: 10.1038/s41467-024-52196-7. Nat Commun. 2024. PMID: 39237586 Free PMC article.
References
-
- Gu L, et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photon. 2019;13:406–411. doi: 10.1038/s41566-019-0408-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

