In vitro modeling of dendritic atrophy in Rett syndrome: determinants for phenotypic drug screening in neurodevelopmental disorders
- PMID: 32051524
- PMCID: PMC7016139
- DOI: 10.1038/s41598-020-59268-w
In vitro modeling of dendritic atrophy in Rett syndrome: determinants for phenotypic drug screening in neurodevelopmental disorders
Abstract
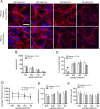
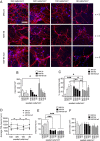
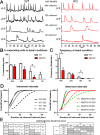
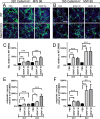
Dendritic atrophy, defined as the reduction in complexity of the neuronal arborization, is a hallmark of several neurodevelopmental disorders, including Rett Syndrome (RTT). RTT, affecting 1:10,000 girls worldwide, is mainly caused by mutations in the MECP2 gene and has no cure. We describe here an in vitro model of dendritic atrophy in Mecp2-/y mouse hippocampal primary cultures, suitable for phenotypic drug-screening. Using High-Content Imaging techniques, we systematically investigated the impact of culturing determinants on several parameters such as neuronal survival, total dendritic length, dendritic endpoints, soma size, cell clusterization, spontaneous activity. Determinants included cell-seeding density, glass or polystyrene substrates, coating with poly-Ornithine with/without Matrigel and miniaturization from 24 to 96-half surface multiwell plates. We show that in all plate-sizes at densities below 320 cells/mm2, morphological parameters remained constant while spontaneous network activity decreased according to the cell-density. Mecp2-/y neurons cultured at 160 cells/mm2 density in 96 multiwell plates, displayed significant dendritic atrophy and showed a marked increase in dendritic length following treatment with Brain-derived neurotrophic factor (BDNF) or Mirtazapine. In conclusion, we have established a phenotypic assay suitable for fast screening of hundreds of compounds, which may be extended to other neurodevelopmental diseases with dendritic atrophy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

