Conserved chromosomal functions of RNA interference
- PMID: 32051563
- PMCID: PMC9478574
- DOI: 10.1038/s41576-019-0203-6
Conserved chromosomal functions of RNA interference
Abstract
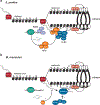
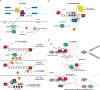
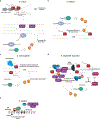
RNA interference (RNAi), a cellular process through which small RNAs target and regulate complementary RNA transcripts, has well-characterized roles in post-transcriptional gene regulation and transposon repression. Recent studies have revealed additional conserved roles for RNAi proteins, such as Argonaute and Dicer, in chromosome function. By guiding chromatin modification, RNAi components promote chromosome segregation during both mitosis and meiosis and regulate chromosomal and genomic dosage response. Small RNAs and the RNAi machinery also participate in the resolution of DNA damage. Interestingly, many of these lesser-studied functions seem to be more strongly conserved across eukaryotes than are well-characterized functions such as the processing of microRNAs. These findings have implications for the evolution of RNAi since the last eukaryotic common ancestor, and they provide a more complete view of the functions of RNAi.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

