TLR9 and beclin 1 crosstalk regulates muscle AMPK activation in exercise
- PMID: 32051584
- PMCID: PMC7047589
- DOI: 10.1038/s41586-020-1992-7
TLR9 and beclin 1 crosstalk regulates muscle AMPK activation in exercise
Abstract
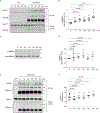
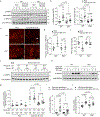
The activation of adenosine monophosphate-activated protein kinase (AMPK) in skeletal muscle coordinates systemic metabolic responses to exercise1. Autophagy-a lysosomal degradation pathway that maintains cellular homeostasis2-is upregulated during exercise, and a core autophagy protein, beclin 1, is required for AMPK activation in skeletal muscle3. Here we describe a role for the innate immune-sensing molecule Toll-like receptor 9 (TLR9)4, and its interaction with beclin 1, in exercise-induced activation of AMPK in skeletal muscle. Mice that lack TLR9 are deficient in both exercise-induced activation of AMPK and plasma membrane localization of the GLUT4 glucose transporter in skeletal muscle, but are not deficient in autophagy. TLR9 binds beclin 1, and this interaction is increased by energy stress (glucose starvation and endurance exercise) and decreased by a BCL2 mutation3,5 that blocks the disruption of BCL2-beclin 1 binding. TLR9 regulates the assembly of the endolysosomal phosphatidylinositol 3-kinase complex (PI3KC3-C2)-which contains beclin 1 and UVRAG-in skeletal muscle during exercise, and knockout of beclin 1 or UVRAG inhibits the cellular AMPK activation induced by glucose starvation. Moreover, TLR9 functions in a muscle-autonomous fashion in ex vivo contraction-induced AMPK activation, glucose uptake and beclin 1-UVRAG complex assembly. These findings reveal a heretofore undescribed role for a Toll-like receptor in skeletal-muscle AMPK activation and glucose metabolism during exercise, as well as unexpected crosstalk between this innate immune sensor and autophagy proteins.
Conflict of interest statement
Figures











Comment in
-
Role for toll-like receptor 9 in muscle AMPK activation.Nat Rev Endocrinol. 2020 Apr;16(4):197. doi: 10.1038/s41574-020-0337-9. Nat Rev Endocrinol. 2020. PMID: 32094467 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

