Mechanical regulation of glycolysis via cytoskeleton architecture
- PMID: 32051585
- PMCID: PMC7210009
- DOI: 10.1038/s41586-020-1998-1
Mechanical regulation of glycolysis via cytoskeleton architecture
Abstract
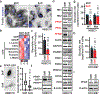
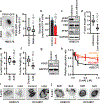
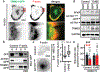
The mechanics of the cellular microenvironment continuously modulates cell functions such as growth, survival, apoptosis, differentiation and morphogenesis via cytoskeletal remodelling and actomyosin contractility1-3. Although all of these processes consume energy4,5, it is unknown whether and how cells adapt their metabolic activity to variable mechanical cues. Here we report that the transfer of human bronchial epithelial cells from stiff to soft substrates causes a downregulation of glycolysis via proteasomal degradation of the rate-limiting metabolic enzyme phosphofructokinase (PFK). PFK degradation is triggered by the disassembly of stress fibres, which releases the PFK-targeting E3 ubiquitin ligase tripartite motif (TRIM)-containing protein 21 (TRIM21). Transformed non-small-cell lung cancer cells, which maintain high glycolytic rates regardless of changing environmental mechanics, retain PFK expression by downregulating TRIM21, and by sequestering residual TRIM21 on a stress-fibre subset that is insensitive to substrate stiffness. Our data reveal a mechanism by which glycolysis responds to architectural features of the actomyosin cytoskeleton, thus coupling cell metabolism to the mechanical properties of the surrounding tissue. These processes enable normal cells to tune energy production in variable microenvironments, whereas the resistance of the cytoskeleton in response to mechanical cues enables the persistence of high glycolytic rates in cancer cells despite constant alterations of the tumour tissue.
Figures










Comment in
-
Stress fibres fuel glycolysis.Nat Rev Mol Cell Biol. 2020 Apr;21(4):180. doi: 10.1038/s41580-020-0224-1. Nat Rev Mol Cell Biol. 2020. PMID: 32060487 No abstract available.
-
Transformed Cells Resist Mechanostimulation's Effects on Metabolism.Cancer Discov. 2020 Apr;10(4):489. doi: 10.1158/2159-8290.CD-RW2020-027. Epub 2020 Feb 21. Cancer Discov. 2020. PMID: 32086316
-
Tension in tumour cells keeps metabolism high.Nature. 2020 Feb;578(7796):517-518. doi: 10.1038/d41586-020-00314-y. Nature. 2020. PMID: 32094916 No abstract available.
-
How to run through mud.Nat Rev Cancer. 2020 Apr;20(4):201. doi: 10.1038/s41568-020-0250-5. Nat Rev Cancer. 2020. PMID: 32127697 No abstract available.
-
Cytoskeleton Architecture Regulates Glycolysis Coupling Cellular Metabolism to Mechanical Cues.Trends Biochem Sci. 2020 Aug;45(8):637-638. doi: 10.1016/j.tibs.2020.04.003. Epub 2020 Apr 25. Trends Biochem Sci. 2020. PMID: 32345468
References
Method References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

