Loss of p53 drives neuron reprogramming in head and neck cancer
- PMID: 32051587
- PMCID: PMC9723538
- DOI: 10.1038/s41586-020-1996-3
Loss of p53 drives neuron reprogramming in head and neck cancer
Abstract
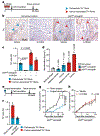
The solid tumour microenvironment includes nerve fibres that arise from the peripheral nervous system1,2. Recent work indicates that newly formed adrenergic nerve fibres promote tumour growth, but the origin of these nerves and the mechanism of their inception are unknown1,3. Here, by comparing the transcriptomes of cancer-associated trigeminal sensory neurons with those of endogenous neurons in mouse models of oral cancer, we identified an adrenergic differentiation signature. We show that loss of TP53 leads to adrenergic transdifferentiation of tumour-associated sensory nerves through loss of the microRNA miR-34a. Tumour growth was inhibited by sensory denervation or pharmacological blockade of adrenergic receptors, but not by chemical sympathectomy of pre-existing adrenergic nerves. A retrospective analysis of samples from oral cancer revealed that p53 status was associated with nerve density, which was in turn associated with poor clinical outcomes. This crosstalk between cancer cells and neurons represents mechanism by which tumour-associated neurons are reprogrammed towards an adrenergic phenotype that can stimulate tumour progression, and is a potential target for anticancer therapy.
Conflict of interest statement
Figures

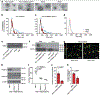




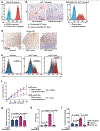


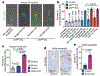
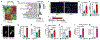
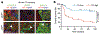
Comment in
-
Loss of p53 protein strikes a nerve to aid tumour growth.Nature. 2020 Feb;578(7795):367-369. doi: 10.1038/d41586-020-00328-6. Nature. 2020. PMID: 32066916 No abstract available.
References
-
- Magnon C et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013). - PubMed
-
- Ayala GE et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 14, 7593–7603 (2008). - PubMed
-
- Schmid JO et al. Cancer cells cue the p53 response of cancer-associated fibroblasts to cisplatin. Cancer Res. 72, 5824–5832 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

