Synthesis, phase transformation, and morphology of hausmannite Mn3O4 nanoparticles: photocatalytic and antibacterial investigations
- PMID: 32051862
- PMCID: PMC7002847
- DOI: 10.1016/j.heliyon.2020.e03245
Synthesis, phase transformation, and morphology of hausmannite Mn3O4 nanoparticles: photocatalytic and antibacterial investigations
Abstract
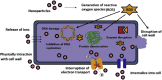
Nano structured Hausmannite (Mn3O4) has efficacious applications in numerous fields, such as catalytic, medical, biosensors, waste water remediation, energy storage devices etc. The potential application in wastewater treatment is due to its distinct structural features combined with fascinating physicochemical properties. Another area of interest is the oxidative properties imparted due to its reduction potential. Larger surface to volume ratio and high reactivity than the bulk form shows great progress as antimicrobial agent to control drug resistant microbial population. The distinct surface morphologies, crystalline forms, reaction conditions and synthetic methods exerts significant impact on the photo catalytic and bactericidal efficiency. Hence, the present paper focuses on a concise review of the multifarious study on synthetic methods of Mn3O4, growth mechanisms, structural forms, phase transformation and phase control, shape and dimensionality. The review also confers its applications towards photo catalytic and bactericidal studies.
Keywords: Antimicrobial activity; Biological sciences; Chemistry; Environmental science; Materials application; Materials chemistry; Materials property; Materials science; Methods of synthesis; Morphology; Nano hausmannite; Nanomaterials; Phase transformation; Photocatalyst.
© 2020 Published by Elsevier Ltd.
Figures





References
-
- Armstrong A.R., Bruce P.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature. 1996;381:499–500.
-
- Bernard M.C., Goff H.L., Thi B.V. Electrochromic reactions in manganese oxides: I. Raman analysis. J. Electrochem. Soc. 1993;140:3065–3070.
-
- Piligkos S., Rajaraman G., Soler Kirchner M., Van Slageren N., Bircher J., Parsons R., Gudel S., Kortus H.U., Wernsdorfer W., Christou G., Brechin E.K. Studies of an enneanuclear manganese single-molecule magnet. J. Am. Chem. Soc. 2005;127:5572. - PubMed
-
- Yoshikai N., Zhang S.L., Yamagata K.I., Tsuji H., Nakamura E. Mechanistic study of the manganese-catalyzed [2 + 2 + 2] annulation of 1,3-dicarbonyl compounds and terminal alkynes. J. Am. Chem. Soc. 2009;131:4099. - PubMed
-
- Reddy A.L.M., Shaijumon M.M., Gowda S.R., Ajayan P.M. Coaxial MnO2/carbon nanotube array electrodes for high-performance lithium batteries. Nano Lett. 2009;9:1002. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

