Age-Related Differences in the Gut Microbiome of Rhesus Macaques
- PMID: 32052009
- PMCID: PMC7302168
- DOI: 10.1093/gerona/glaa048
Age-Related Differences in the Gut Microbiome of Rhesus Macaques
Abstract
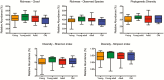
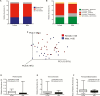
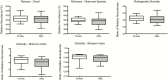
Aging is a multifactorial process characterized by progressive changes in gut physiology and the intestinal mucosal immune system. These changes, along with alterations in lifestyle, diet, nutrition, inflammation and immune function alter both composition and stability of the gut microbiota. Given the impact of environmental influences on the gut microbiota, animal models are particularly useful in this field. To understand the relationship between the gut microbiota and aging in nonhuman primates, we collected fecal samples from 20 male and 20 female rhesus macaques (Macaca mulatta), across the natural macaque age range, for 16S rRNA gene analyses. Operational taxonomic units were then grouped together to summarize taxon abundance at different hierarchical levels of classification and alpha- and beta-diversity were calculated. There were no age or sex differences in alpha diversity. At the phylum level, relative abundance of Proteobacteria and Firmicutes and Firmicutes to Bacteriodetes ratio were different between age groups though significance disappeared after correction for multiple comparisons. At the class level, relative abundance of Firmicutes_Bacilli decreased and Proteobacteria_Alphaproteobacteria and Proteobacteria_Betaproteobacteria increased with each successively older group. Only differences in Firmicutes_Bacilli remained significant after correction for multiple comparisons. No sex differences were identified in relative abundances after correction for multiple comparisons. Our results are not surprising given the known impact of environmental factors on the gut microbiota.
Keywords: Animal model; Biological age; Frailty; Monkey.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Characterization of the Gut Microbiota in Six Geographical Populations of Chinese Rhesus Macaques (Macaca mulatta), Implying an Adaptation to High-Altitude Environment.Microb Ecol. 2018 Aug;76(2):565-577. doi: 10.1007/s00248-018-1146-8. Epub 2018 Jan 25. Microb Ecol. 2018. PMID: 29372281
-
Characterizing the fecal microbiota of infants with botulism.Microbiome. 2015 Nov 23;3:54. doi: 10.1186/s40168-015-0119-0. Microbiome. 2015. PMID: 26593441 Free PMC article.
-
Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease.Nutrients. 2016 Oct 28;8(11):684. doi: 10.3390/nu8110684. Nutrients. 2016. PMID: 27801835 Free PMC article.
-
Alcohol-induced changes in the gut microbiome and metabolome of rhesus macaques.Psychopharmacology (Berl). 2019 May;236(5):1531-1544. doi: 10.1007/s00213-019-05217-z. Epub 2019 Mar 22. Psychopharmacology (Berl). 2019. PMID: 30903211 Free PMC article.
-
The Gut Microbiome, Aging, and Longevity: A Systematic Review.Nutrients. 2020 Dec 7;12(12):3759. doi: 10.3390/nu12123759. Nutrients. 2020. PMID: 33297486 Free PMC article.
Cited by
-
Age and sex-associated variation in the multi-site microbiome of an entire social group of free-ranging rhesus macaques.Microbiome. 2021 Mar 22;9(1):68. doi: 10.1186/s40168-021-01009-w. Microbiome. 2021. PMID: 33752735 Free PMC article.
-
Gut dysbiosis and age-related neurological diseases; an innovative approach for therapeutic interventions.Transl Res. 2020 Dec;226:39-56. doi: 10.1016/j.trsl.2020.07.012. Epub 2020 Aug 2. Transl Res. 2020. PMID: 32755639 Free PMC article. Review.
-
Fecal bacterial communities of wild black capuchin monkeys (Sapajus nigritus) from the Atlantic Forest biome in Southern Brazil are divergent from those of other non-human primates.Curr Res Microb Sci. 2021 Jul 10;2:100048. doi: 10.1016/j.crmicr.2021.100048. eCollection 2021 Dec. Curr Res Microb Sci. 2021. PMID: 34841339 Free PMC article.
-
Geographical distribution and species variation of gut microbiota in small rodents from the agro-pastoral transition ecotone in northern China.Ecol Evol. 2024 Mar 11;14(3):e11084. doi: 10.1002/ece3.11084. eCollection 2024 Mar. Ecol Evol. 2024. PMID: 38469048 Free PMC article.
-
Young at Gut-Turning Back the Clock with the Gut Microbiome.Microorganisms. 2021 Mar 8;9(3):555. doi: 10.3390/microorganisms9030555. Microorganisms. 2021. PMID: 33800340 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

