Inhibiting DosRST as a new approach to tuberculosis therapy
- PMID: 32053005
- PMCID: PMC7607383
- DOI: 10.4155/fmc-2019-0263
Inhibiting DosRST as a new approach to tuberculosis therapy
Abstract
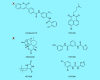
Progress against tuberculosis (TB) requires faster-acting drugs. Mycobacterium tuberculosis (Mtb) is the leading cause of death by an infectious disease and its treatment is challenging and lengthy. Mtb is remarkably successful, in part, due to its ability to become dormant in response to host immune pressures. The DosRST two-component regulatory system is induced by hypoxia, nitric oxide and carbon monoxide and remodels Mtb physiology to promote nonreplicating persistence (NRP). NRP bacteria are thought to play a role in the long course of TB treatment. Therefore, inhibitors of DosRST-dependent adaptation may function to kill this reservoir of persisters and potentially shorten therapy. This review examines the function of DosRST, newly discovered compounds that inhibit DosRST signaling and considers future development of DosRST inhibitors as adjunct therapies.
Keywords: antivirulence therapies; drug discovery and development; drug tolerance; tuberculosis; two-component regulatory systems.
Conflict of interest statement
This project was supported by the Bill and Melinda Gates Foundation (grant no. OPP1059227 and OPP1119065) and the National Institute of Allergy and Infectious Diseases (grant no. R01AI116605 and R21AI105867). RB Abramovitch is the founder of Tarn Biosciences, a company developing new TB drugs. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
