A large-scale resource for tissue-specific CRISPR mutagenesis in Drosophila
- PMID: 32053108
- PMCID: PMC7062466
- DOI: 10.7554/eLife.53865
A large-scale resource for tissue-specific CRISPR mutagenesis in Drosophila
Abstract
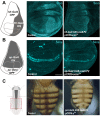
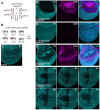
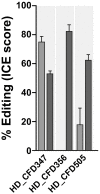
Genetic screens are powerful tools for the functional annotation of genomes. In the context of multicellular organisms, interrogation of gene function is greatly facilitated by methods that allow spatial and temporal control of gene abrogation. Here, we describe a large-scale transgenic short guide (sg) RNA library for efficient CRISPR-based disruption of specific target genes in a constitutive or conditional manner. The library consists currently of more than 2600 plasmids and 1700 fly lines with a focus on targeting kinases, phosphatases and transcription factors, each expressing two sgRNAs under control of the Gal4/UAS system. We show that conditional CRISPR mutagenesis is robust across many target genes and can be efficiently employed in various somatic tissues, as well as the germline. In order to prevent artefacts commonly associated with excessive amounts of Cas9 protein, we have developed a series of novel UAS-Cas9 transgenes, which allow fine tuning of Cas9 expression to achieve high gene editing activity without detectable toxicity. Functional assays, as well as direct sequencing of genomic sgRNA target sites, indicates that the vast majority of transgenic sgRNA lines mediate efficient gene disruption. Furthermore, we conducted the so far largest fully transgenic CRISPR screen in any metazoan organism, which further supported the high efficiency and accuracy of our library and revealed many so far uncharacterized genes essential for development.
Keywords: CRISPR; D. melanogaster; Resource; conditional mutagenesis; developmental biology; genetic tools; genetics; genomics; large-scale in vivo screens.
Plain language summary
Twenty years after the release of the sequence of the human genome, the role of many genes is still unknown. This is partly because some of these genes may only be active in specific types of cells or for short periods of time, which makes them difficult to study. A powerful way to gather information about human genes is to examine their equivalents in ‘model’ animals such as fruit flies. Researchers can use genetic methods to create strains of insects where genes are deactivated; evaluating the impact of these manipulations on the animals helps to understand the roles of the defunct genes. However, the current methods struggle to easily delete target genes, especially only in certain cells, or at precise times. Here, Port et al. genetically engineered flies that carry CRISPR-Cas9, a biological system that can be programmed to ‘cut’ and mutate precise genetic sequences. The insects were also manipulated in such a way that the CRISPR elements could be switched on at will, and their quantity finely tuned. This work resulted in a collection of more than 1,700 fruit fly strains in which specific genes could be deactivated on demand in precise cells. Further experiments confirmed that this CRISPR system could mutate target genes in different parts of the fly, including in the eyes, gut and wings. Port et al. have made their collection of genetically engineered fruit flies publically available, so that other researchers can use the strains in their experiments. The CRISPR technology they refined and developed may also lay the foundation for similar collections in other model organisms.
© 2020, Port et al.
Conflict of interest statement
FP, CS, MS, BR, FH, JZ, CB, JF, AH, KK, LL, NL, RM, BP, KR, LS, LV, EV, MB No competing interests declared
Figures







References
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

