Anti-platelet antibodies in childhood immune thrombocytopenia: Prevalence and prognostic implications
- PMID: 32053276
- PMCID: PMC7318215
- DOI: 10.1111/jth.14762
Anti-platelet antibodies in childhood immune thrombocytopenia: Prevalence and prognostic implications
Abstract
Background: Anti-platelet antibody testing may be useful for the diagnosis and management of childhood immune thrombocytopenia (ITP).
Objectives: Here we aimed to assess the prevalence and prognostic significance of anti-platelet glycoprotein-specific IgM and IgG antibodies.
Methods: Children with newly diagnosed ITP were included at diagnosis and randomized to an intravenous immunoglobulins (IVIg) or careful observation group (TIKI trial). In this well-defined and longitudinally followed cohort (N = 179), anti-platelet glycoprotein-specific IgM and IgG antibodies were determined by monoclonal antibody-immobilization of platelet antigens.
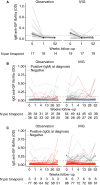
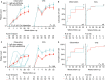
Results: The dominant circulating anti-platelet antibody class in childhood ITP was IgM (62% of patients); but IgG antibodies were also found (10%). Children without IgM platelet antibodies were older and more often female. There was weak evidence for an association between IgM anti-GP IIb/IIIa antibodies and an increased bleeding severity (P = .03). The IgM and IgG anti-platelet responses partially overlapped, and reactivity was frequently directed against multiple glycoproteins. During 1-year follow-up, children with IgM antibodies in the observation group displayed a faster platelet recovery compared to children without, also after adjustment for age and preceding infections (P = 7.1 × 10-5 ). The small group of patients with detectable IgG anti-platelet antibodies exhibited an almost complete response to IVIg treatment (N = 12; P = .02), suggesting that IVIg was particularly efficacious in these children.
Conclusions: Testing for circulating anti-platelet antibodies may be helpful for the clinical prognostication and the guidance of treatment decisions in newly diagnosed childhood ITP. Our data suggest that the development of even more sensitive tests may further improve the clinical value of antibody testing.
Keywords: autoantibodies; immune thrombocytopenia; intravenous immunoglobulins; pediatrics; platelets.
© 2019 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals, Inc. on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
DES, KMJHP, MCAB, LP, CEvdS, GV, and MdH declare no competing financial interests.
Figures


References
-
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174‐180. - PubMed
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. - PubMed
-
- Vanderlugt CL, Miller SD. Epitope spreading in immune‐mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2(2):85‐95. - PubMed
-
- Heitink‐Polle KMJ, Nijsten J, Boonacker CWB, de Haas M, Bruin MCA. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta‐analysis. Blood. 2014;124(22):3295‐3307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

