Rational Design of Mechanism-Based Inhibitors and Activity-Based Probes for the Identification of Retaining α-l-Arabinofuranosidases
- PMID: 32053363
- PMCID: PMC7068720
- DOI: 10.1021/jacs.9b11351
Rational Design of Mechanism-Based Inhibitors and Activity-Based Probes for the Identification of Retaining α-l-Arabinofuranosidases
Abstract
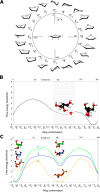
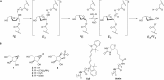
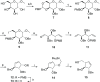
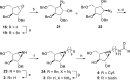
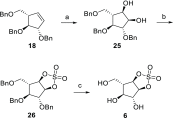
Identifying and characterizing the enzymes responsible for an observed activity within a complex eukaryotic catabolic system remains one of the most significant challenges in the study of biomass-degrading systems. The debranching of both complex hemicellulosic and pectinaceous polysaccharides requires the production of α-l-arabinofuranosidases among a wide variety of coexpressed carbohydrate-active enzymes. To selectively detect and identify α-l-arabinofuranosidases produced by fungi grown on complex biomass, potential covalent inhibitors and probes which mimic α-l-arabinofuranosides were sought. The conformational free energy landscapes of free α-l-arabinofuranose and several rationally designed covalent α-l-arabinofuranosidase inhibitors were analyzed. A synthetic route to these inhibitors was subsequently developed based on a key Wittig-Still rearrangement. Through a combination of kinetic measurements, intact mass spectrometry, and structural experiments, the designed inhibitors were shown to efficiently label the catalytic nucleophiles of retaining GH51 and GH54 α-l-arabinofuranosidases. Activity-based probes elaborated from an inhibitor with an aziridine warhead were applied to the identification and characterization of α-l-arabinofuranosidases within the secretome of A. niger grown on arabinan. This method was extended to the detection and identification of α-l-arabinofuranosidases produced by eight biomass-degrading basidiomycete fungi grown on complex biomass. The broad applicability of the cyclophellitol-derived activity-based probes and inhibitors presented here make them a valuable new tool in the characterization of complex eukaryotic carbohydrate-degrading systems and in the high-throughput discovery of α-l-arabinofuranosidases.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Hekmat O.; Kim Y. W.; Williams S. J.; He S.; Withers S. G. Active-Site Peptide “Fingerprinting” of Glycosidases in Complex Mixtures by Mass Spectrometry: Discovery of a Novel Retaining β-1,4-Glycanase in Cellulomonas Fimi. J. Biol. Chem. 2005, 280 (42), 35126–35135. 10.1074/jbc.M508434200. - DOI - PubMed

