Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020
- PMID: 32053584
- PMCID: PMC7041302
- DOI: 10.15585/mmwr.rr6901a1
Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020
Abstract
Comprehensive guidelines for treatment of latent tuberculosis infection (LTBI) among persons living in the United States were last published in 2000 (American Thoracic Society. CDC targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221-47). Since then, several new regimens have been evaluated in clinical trials. To update previous guidelines, the National Tuberculosis Controllers Association (NTCA) and CDC convened a committee to conduct a systematic literature review and make new recommendations for the most effective and least toxic regimens for treatment of LTBI among persons who live in the United States.The systematic literature review included clinical trials of regimens to treat LTBI. Quality of evidence (high, moderate, low, or very low) from clinical trial comparisons was appraised using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria. In addition, a network meta-analysis evaluated regimens that had not been compared directly in clinical trials. The effectiveness outcome was tuberculosis disease; the toxicity outcome was hepatotoxicity. Strong GRADE recommendations required at least moderate evidence of effectiveness and that the desirable consequences outweighed the undesirable consequences in the majority of patients. Conditional GRADE recommendations were made when determination of whether desirable consequences outweighed undesirable consequences was uncertain (e.g., with low-quality evidence).These updated 2020 LTBI treatment guidelines include the NTCA- and CDC-recommended treatment regimens that comprise three preferred rifamycin-based regimens and two alternative monotherapy regimens with daily isoniazid. All recommended treatment regimens are intended for persons infected with Mycobacterium tuberculosis that is presumed to be susceptible to isoniazid or rifampin. These updated guidelines do not apply when evidence is available that the infecting M. tuberculosis strain is resistant to both isoniazid and rifampin; recommendations for treating contacts exposed to multidrug-resistant tuberculosis were published in 2019 (Nahid P, Mase SR Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2019;200:e93-e142). The three rifamycin-based preferred regimens are 3 months of once-weekly isoniazid plus rifapentine, 4 months of daily rifampin, or 3 months of daily isoniazid plus rifampin. Prescribing providers or pharmacists who are unfamiliar with rifampin and rifapentine might confuse the two drugs. They are not interchangeable, and caution should be taken to ensure that patients receive the correct medication for the intended regimen. Preference for these rifamycin-based regimens was made on the basis of effectiveness, safety, and high treatment completion rates. The two alternative treatment regimens are daily isoniazid for 6 or 9 months; isoniazid monotherapy is efficacious but has higher toxicity risk and lower treatment completion rates than shorter rifamycin-based regimens.In summary, short-course (3- to 4-month) rifamycin-based treatment regimens are preferred over longer-course (6-9 month) isoniazid monotherapy for treatment of LTBI. These updated guidelines can be used by clinicians, public health officials, policymakers, health care organizations, and other state and local stakeholders who might need to adapt them to fit individual clinical circumstances.
Conflict of interest statement
All authors, who are also the LTBI treatment guidelines committee members, have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
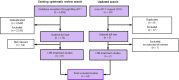
References
-
- Sutherland I. The ten-year incidence of clinical TB following conversion in 2,550 individuals aged 14 to 19 years. Tuberculosis Surveillance Research Unit Progress Report. The Hague, the Netherlands: The Royal Netherlands Tuberculosis Foundation; 1968.
-
- Sutherland I. Recent studies in the epidemiology of tuberculosis, based on the risk of being infected with tubercle bacilli. Adv Tuberc Res 1976;19:1–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
