In-silico simulated prototype-patients using TPMS technology to study a potential adverse effect of sacubitril and valsartan
- PMID: 32053711
- PMCID: PMC7018085
- DOI: 10.1371/journal.pone.0228926
In-silico simulated prototype-patients using TPMS technology to study a potential adverse effect of sacubitril and valsartan
Abstract
Unveiling the mechanism of action of a drug is key to understand the benefits and adverse reactions of a medication in an organism. However, in complex diseases such as heart diseases there is not a unique mechanism of action but a wide range of different responses depending on the patient. Exploring this collection of mechanisms is one of the clues for a future personalized medicine. The Therapeutic Performance Mapping System (TPMS) is a Systems Biology approach that generates multiple models of the mechanism of action of a drug. Each molecular mechanism generated could be associated to particular individuals, here defined as prototype-patients, hence the generation of models using TPMS technology may be used for detecting adverse effects to specific patients. TPMS operates by (1) modelling the responses in humans with an accurate description of a protein network and (2) applying a Multilayer Perceptron-like and sampling strategy to find all plausible solutions. In the present study, TPMS is applied to explore the diversity of mechanisms of action of the drug combination sacubitril/valsartan. We use TPMS to generate a wide range of models explaining the relationship between sacubitril/valsartan and heart failure (the indication), as well as evaluating their association with macular degeneration (a potential adverse effect). Among the models generated, we identify a set of mechanisms of action associated to a better response in terms of heart failure treatment, which could also be associated to macular degeneration development. Finally, a set of 30 potential biomarkers are proposed to identify mechanisms (or prototype-patients) more prone of suffering macular degeneration when presenting good heart failure response. All prototype-patients models generated are completely theoretical and therefore they do not necessarily involve clinical effects in real patients. Data and accession to software are available at http://sbi.upf.edu/data/tpms/.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Baldo Oliva, currently serves on the editorial board as academic editor of PLOS ONE. The commercial affiliation (Anaxomics Biotech SL) of the authors does not alter our adherence to PLOS ONE policies on sharing data and materials
Figures
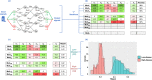

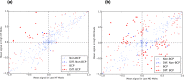
Similar articles
-
Effect of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients With HFpEF.J Am Coll Cardiol. 2020 Aug 4;76(5):503-514. doi: 10.1016/j.jacc.2020.05.072. J Am Coll Cardiol. 2020. PMID: 32731928 Clinical Trial.
-
Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial.JAMA. 2021 Nov 16;326(19):1919-1929. doi: 10.1001/jama.2021.18463. JAMA. 2021. PMID: 34783839 Free PMC article. Clinical Trial.
-
Synergy between sacubitril and valsartan leads to hemodynamic, antifibrotic, and exercise tolerance benefits in rats with preexisting heart failure.Am J Physiol Heart Circ Physiol. 2019 Feb 1;316(2):H289-H297. doi: 10.1152/ajpheart.00579.2018. Epub 2018 Nov 21. Am J Physiol Heart Circ Physiol. 2019. PMID: 30461302
-
The safety of sacubitril-valsartan for the treatment of chronic heart failure.Expert Opin Drug Saf. 2017 Feb;16(2):257-263. doi: 10.1080/14740338.2017.1279144. Expert Opin Drug Saf. 2017. PMID: 28060547 Review.
-
Sacubitril/Valsartan: The Newest Addition to the Toolbox for Guideline-Directed Medical Therapy of Heart Failure.Am J Med. 2017 Jun;130(6):635-639. doi: 10.1016/j.amjmed.2017.02.010. Epub 2017 Mar 9. Am J Med. 2017. PMID: 28285069 Review.
Cited by
-
Dendritic Cells and Microglia Have Non-redundant Functions in the Inflamed Brain with Protective Effects of Type 1 cDCs.Cell Rep. 2020 Oct 20;33(3):108291. doi: 10.1016/j.celrep.2020.108291. Cell Rep. 2020. PMID: 33086061 Free PMC article.
-
Ceruletide and Alpha-1 Antitrypsin as a Novel Combination Therapy for Ischemic Stroke.Neurotherapeutics. 2022 Mar;19(2):513-527. doi: 10.1007/s13311-022-01203-0. Epub 2022 Feb 28. Neurotherapeutics. 2022. PMID: 35226340 Free PMC article.
-
Artificial intelligence-driven drug repurposing and structural biology for SARS-CoV-2.Curr Res Pharmacol Drug Discov. 2021;2:100042. doi: 10.1016/j.crphar.2021.100042. Epub 2021 Jul 28. Curr Res Pharmacol Drug Discov. 2021. PMID: 34870150 Free PMC article. Review.
-
A decision support system based on artificial intelligence and systems biology for the simulation of pancreatic cancer patient status.CPT Pharmacometrics Syst Pharmacol. 2023 Jul;12(7):916-928. doi: 10.1002/psp4.12961. Epub 2023 Mar 31. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 37002678 Free PMC article.
-
Application of systems biology to identify pharmacological mechanisms of thrombotic microangiopathy evoked by combined activated prothrombin complex concentrate and emicizumab.Sci Rep. 2023 Jun 21;13(1):10078. doi: 10.1038/s41598-023-36891-x. Sci Rep. 2023. PMID: 37344529 Free PMC article.
References
-
- Viceconti M, Clapworthy G. The virtual physiological human: Challenges and opportunities. 2006 3rd IEEE International Symposium on Biomedical Imaging: From Nano to Macro—Proceedings. 2006. pp. 812–815. 10.1109/isbi.2006.1625042 - DOI
-
- Viceconti M, Henney A, Morley-Fletcher E. In silico clinical trials: how computer simulation will transform the biomedical industry. Int J Clin Trials. 2016;3: 37 10.18203/2349-3259.ijct20161408 - DOI
-
- Anaxomics Biotech SL. TPMS technology [Internet]. 2018. Available: http://www.anaxomics.com/tpms.php
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

